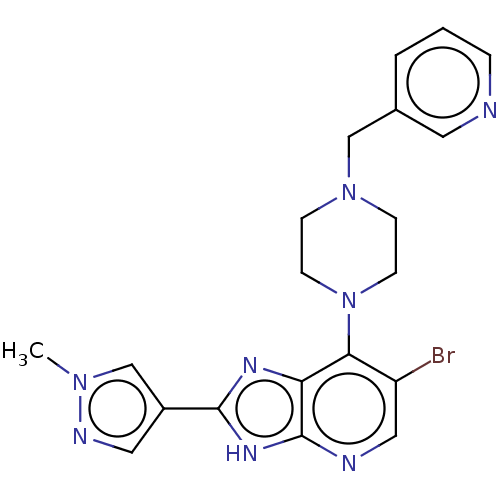
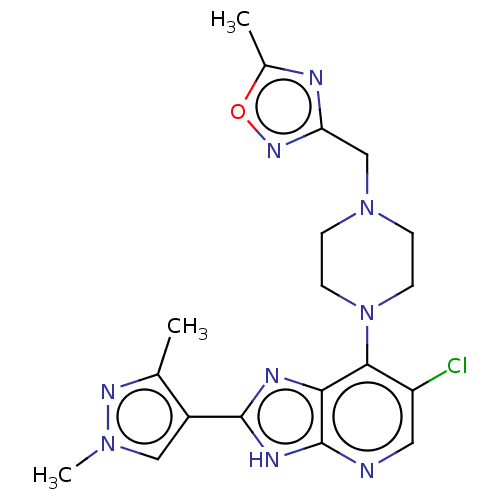
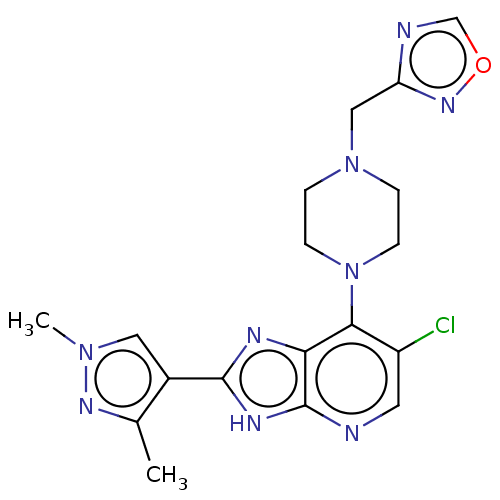
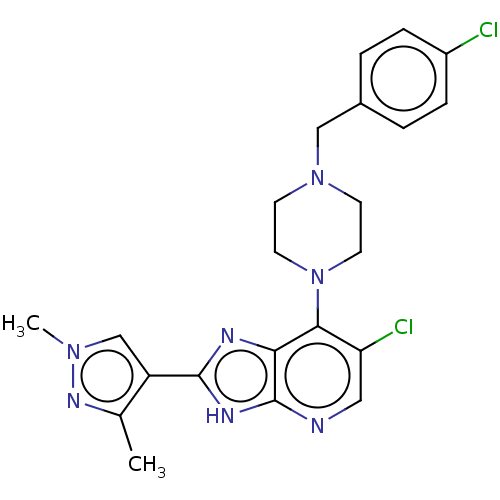
Affinity DataIC50: 5.5nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with...More data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with...More data for this Ligand-Target Pair
Affinity DataIC50: 52nMAssay Description:Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Myc-tagged Aurora A was transfected in Hela cells using Lipofectamine LTX in 24 well plates, and 24 hours after transfection, cells were treated with...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataIC50: 5.50E+3nMAssay Description:All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataIC50: 9.50E+3nMAssay Description:All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataIC50: 1.08E+4nMAssay Description:All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataIC50: 1.10E+4nMAssay Description:All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataIC50: 2.50E+4nMAssay Description:All hERG percentage inhibitions at 10 uM compound concentration were determined by Millipore in a high-throughput cell-based electrophysiology assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3 [K663Q](Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataKd: 5.10nMAssay Description:The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3 [D835Y](Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataKd: 14nMAssay Description:The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3 [D835H](Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataKd: 11nMAssay Description:The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataKd: 6.20nMAssay Description:The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K...More data for this Ligand-Target Pair
Affinity DataKd: 48nMAssay Description:The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3 [N841I](Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataKd: 16nMAssay Description:The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K...More data for this Ligand-Target Pair
Affinity DataKd: 7.5nMAssay Description:The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3 [R834Q](Homo sapiens (Human))
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
Affinity DataKd: 110nMAssay Description:The kinase selectivity was assessed by profiling Example 1 in a 442-kinase panel (386 non-mutant kinases) at a concentration of 1 μM using the K...More data for this Ligand-Target Pair