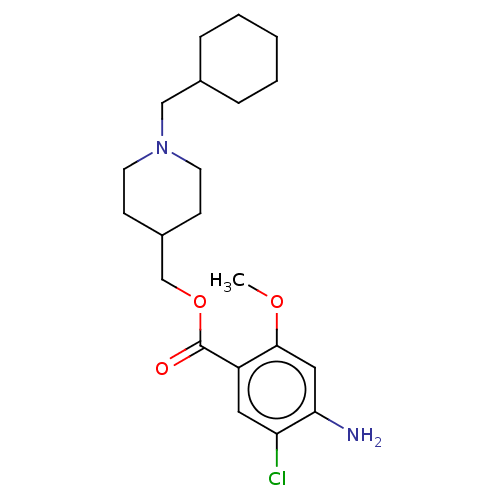
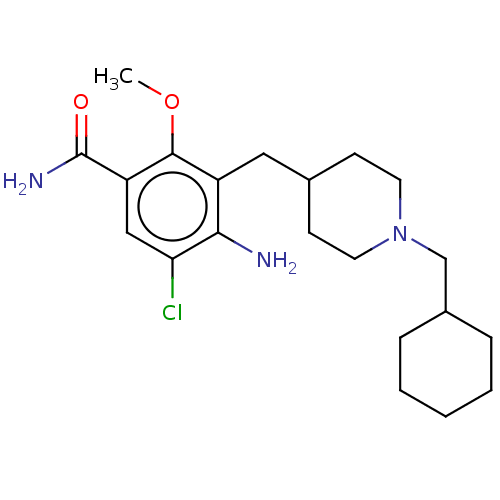
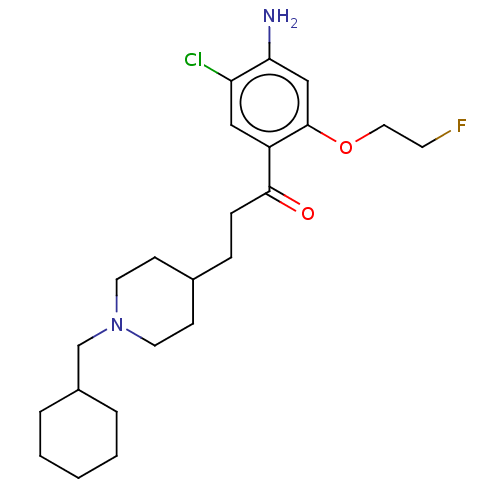
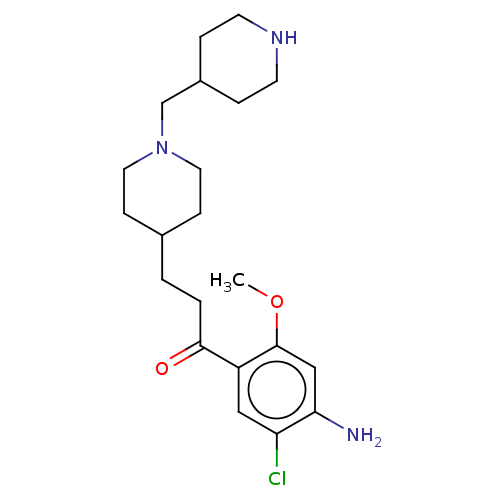
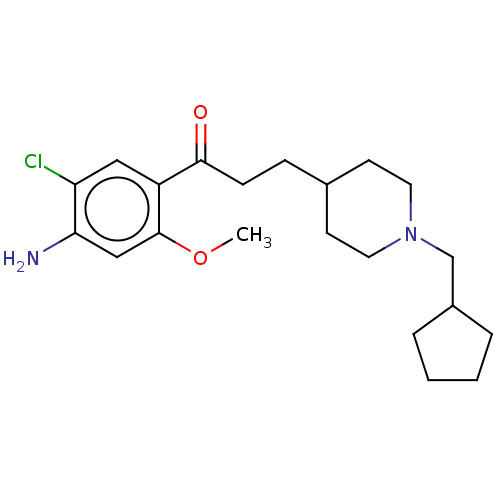
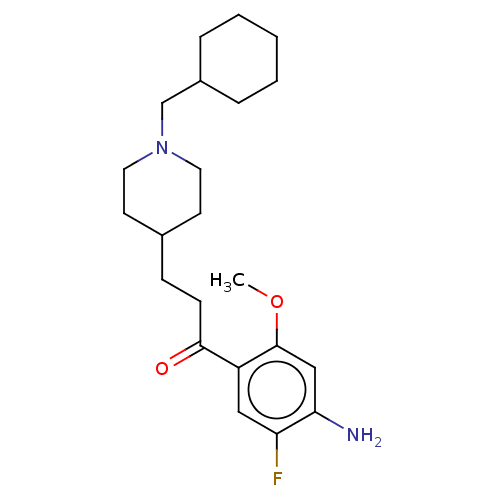
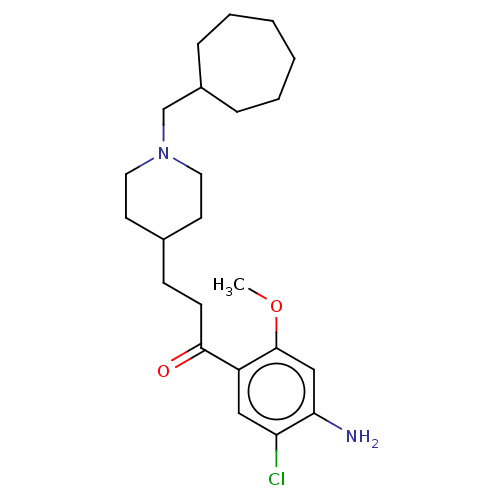
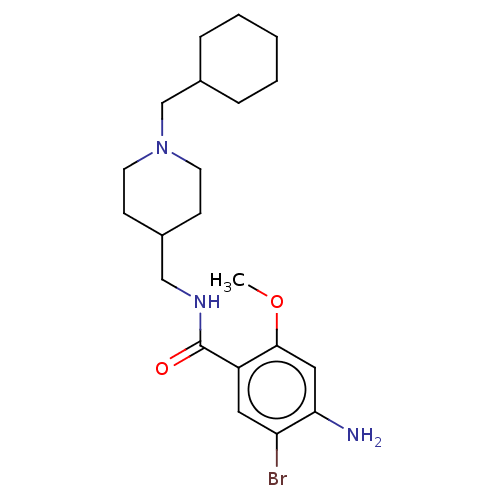
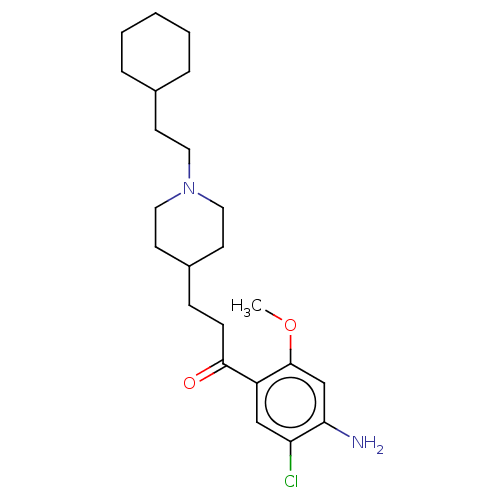
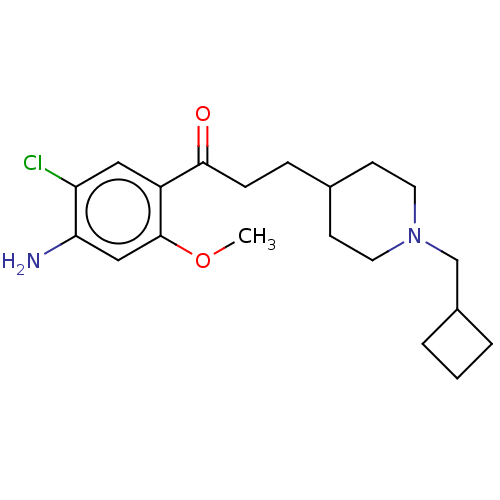
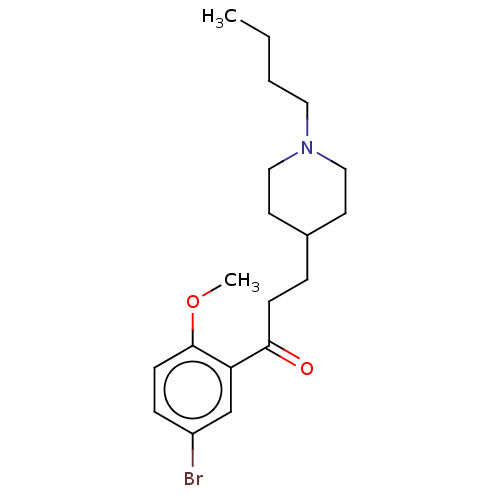
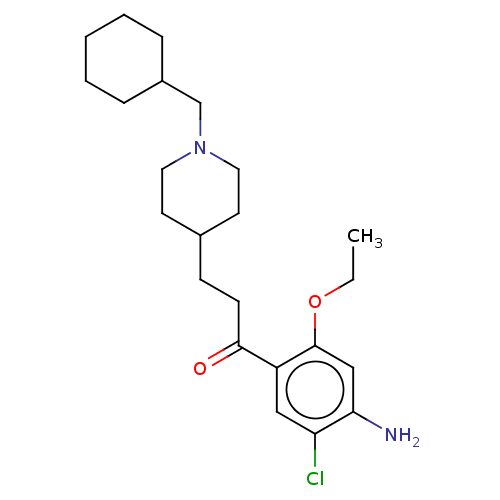
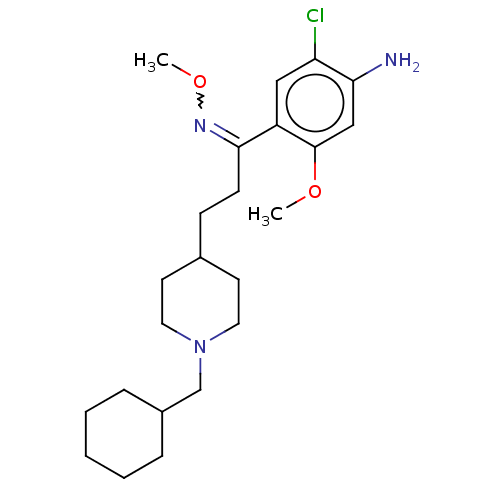
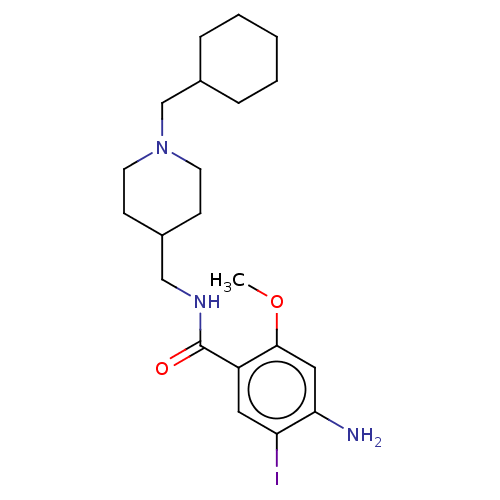
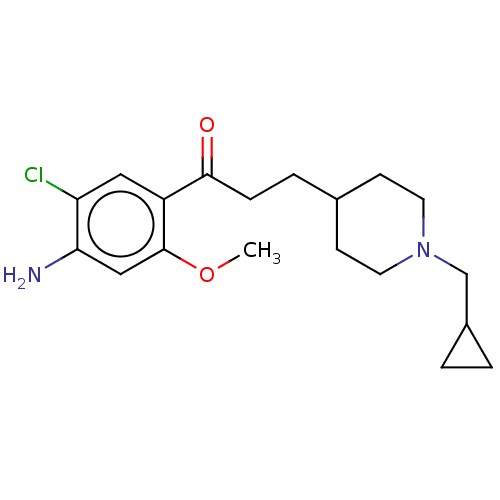
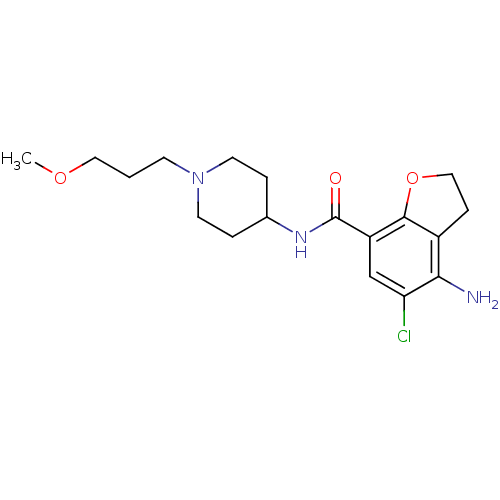
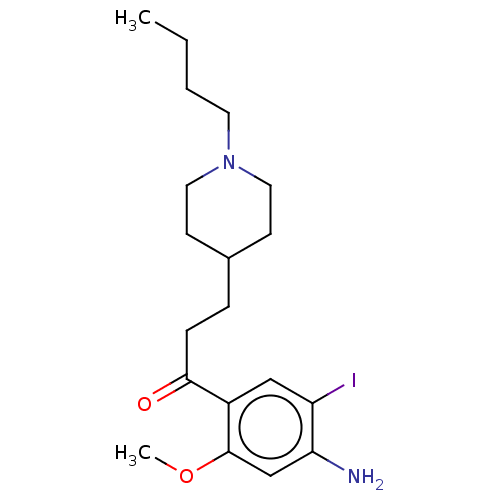
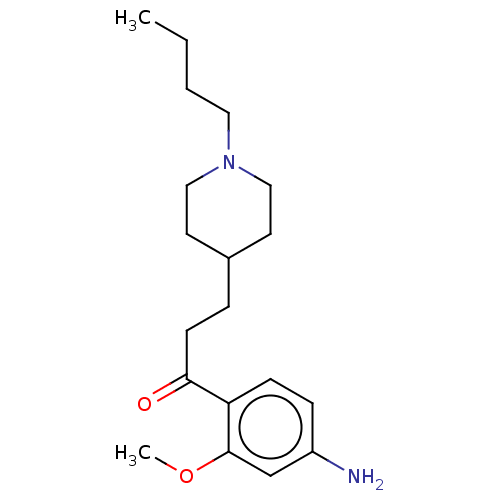
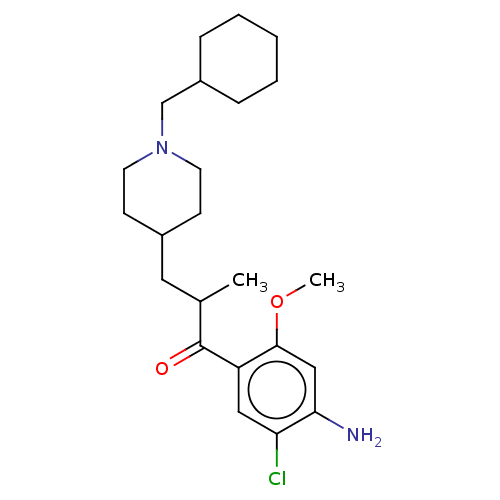
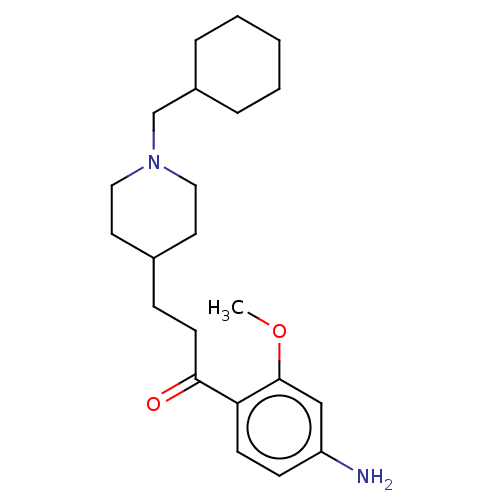
Affinity DataKi: 0.900nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 3.80nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 3.90nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 5.40nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 7.10nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 8.20nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 8.80nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 12.5nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 13.1nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 13.9nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 14.8nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 18.1nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 44.1nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 44.7nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 88.6nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 125nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 125nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair
Affinity DataKi: 125nMAssay Description:Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an...More data for this Ligand-Target Pair