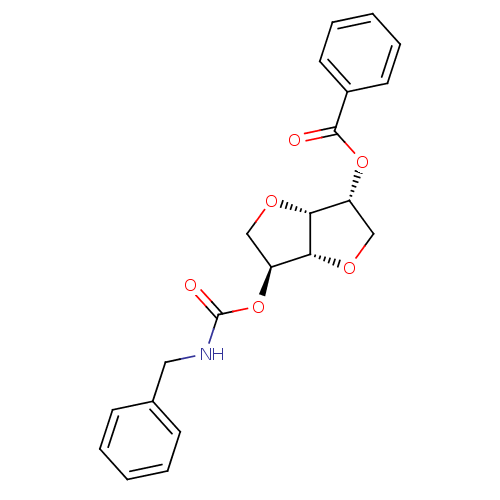
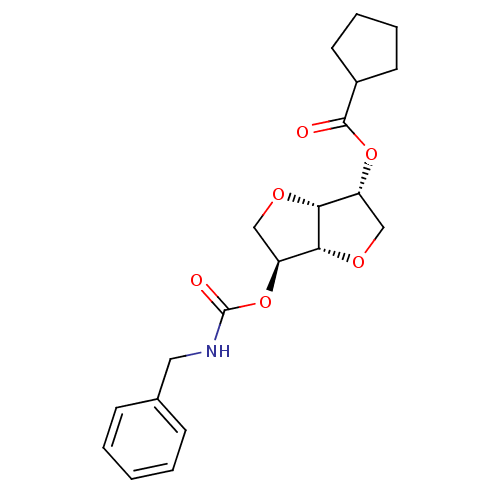
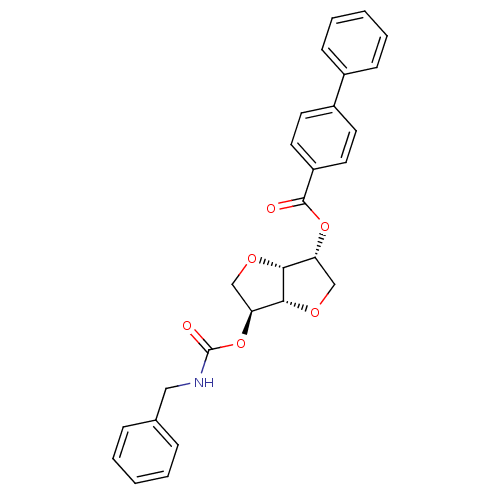
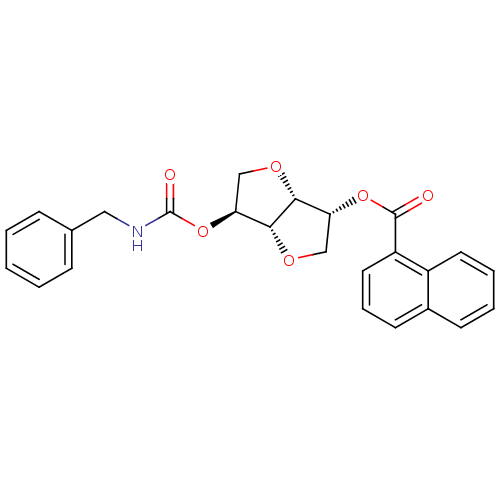
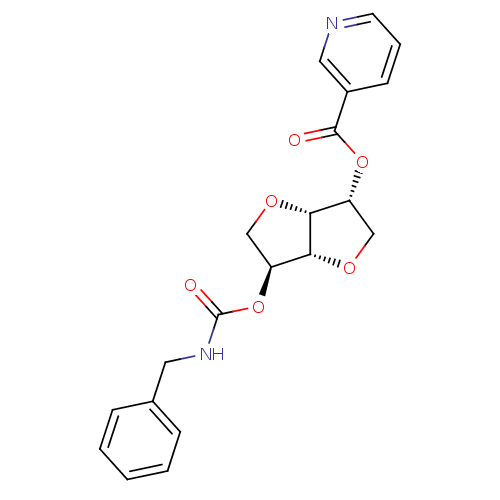
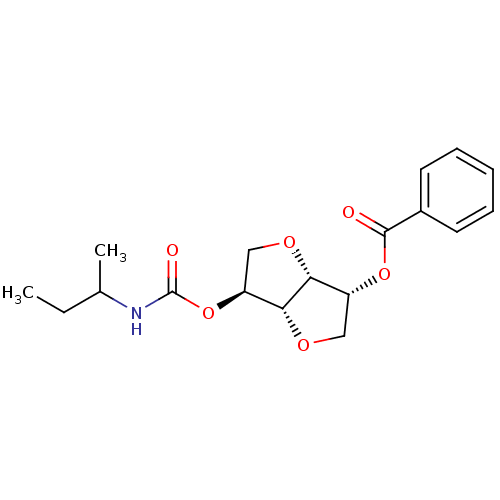
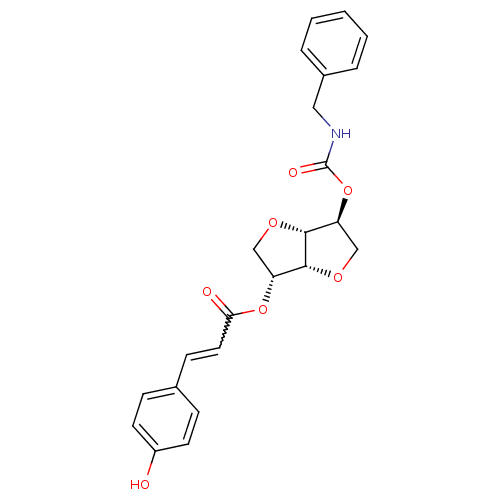
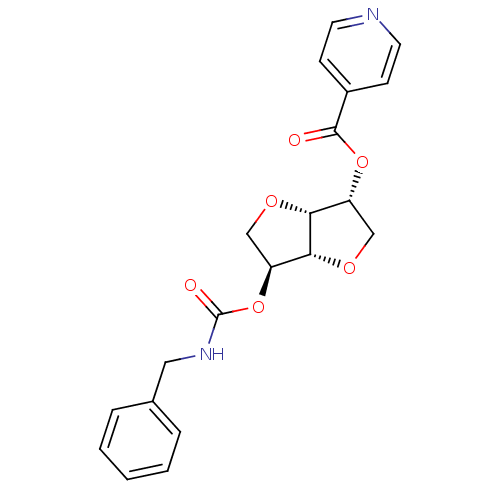
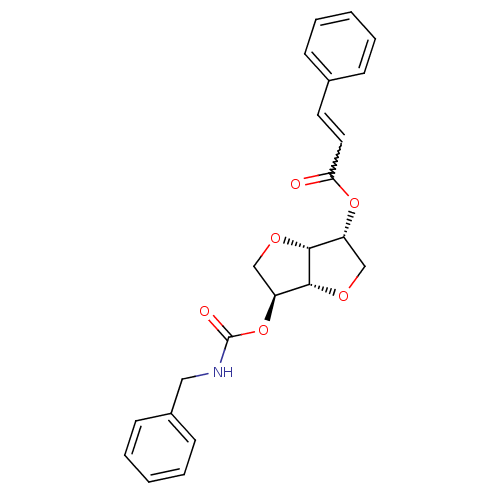
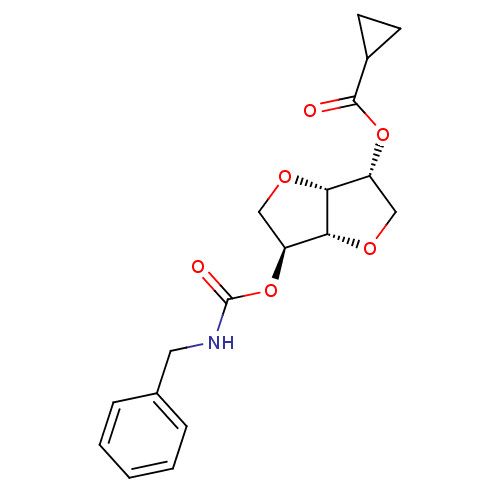
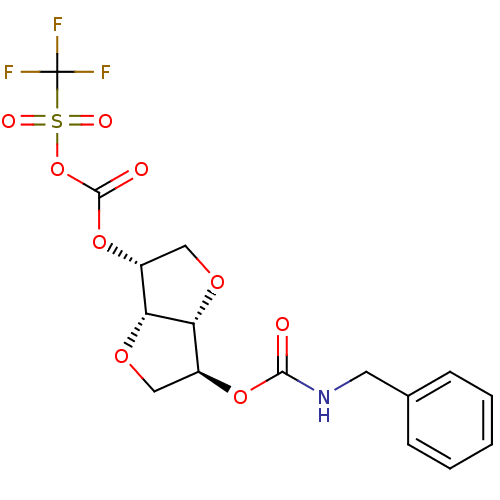
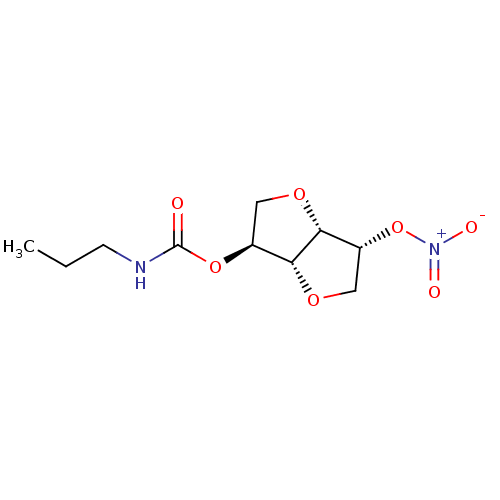
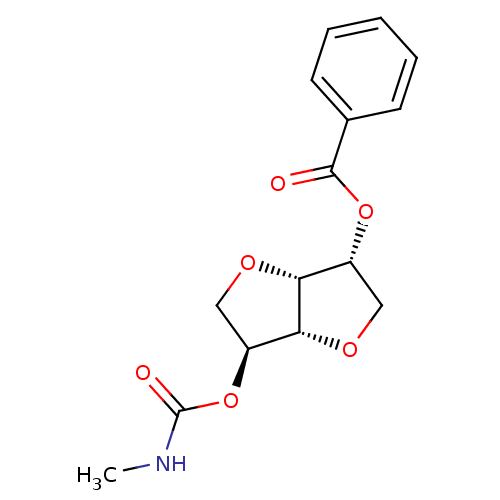
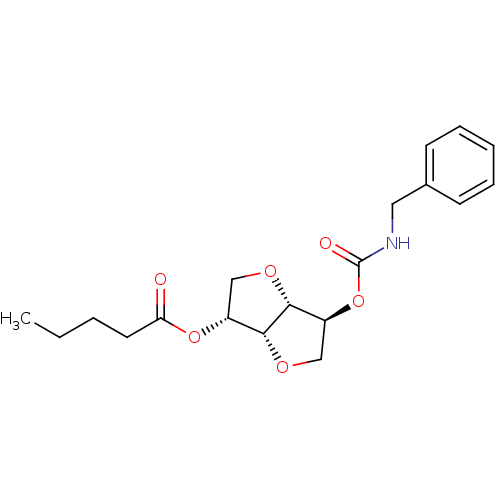
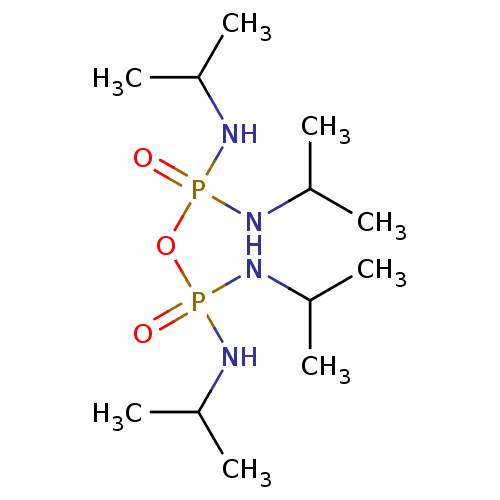
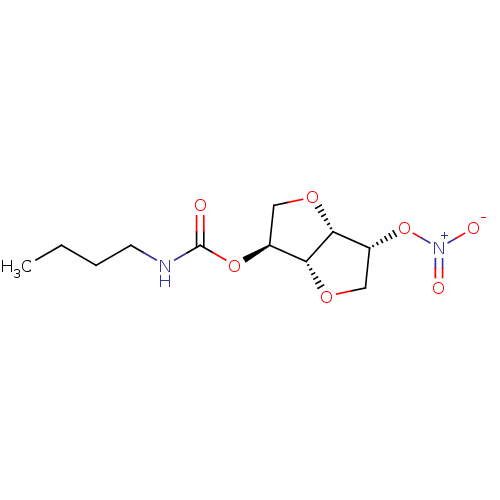
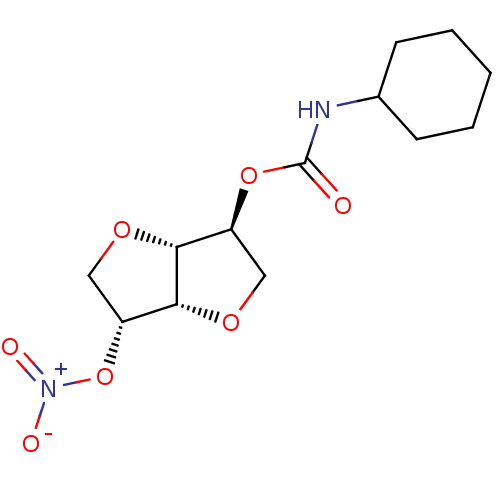
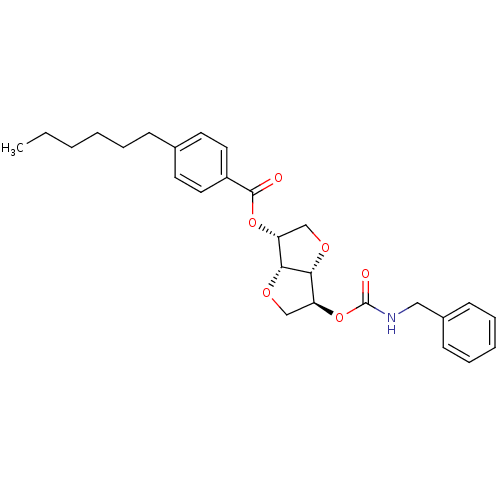
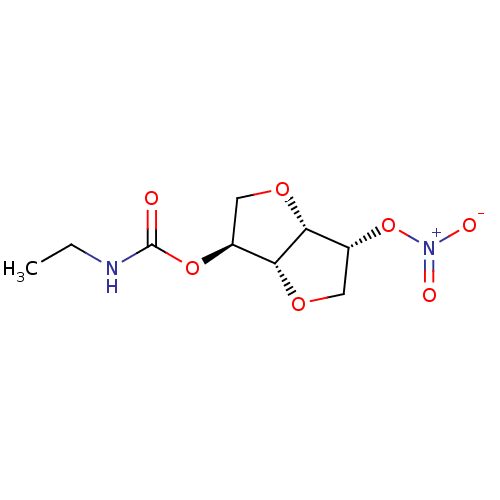
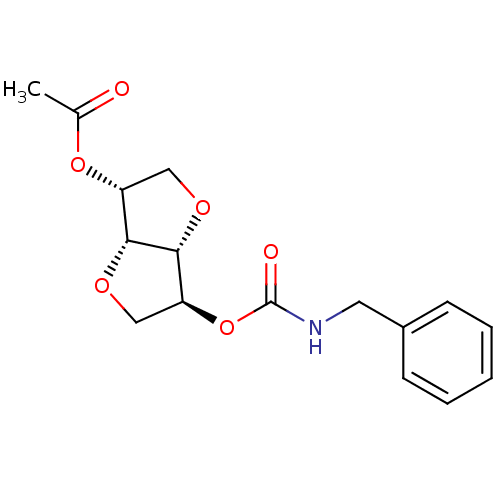
Affinity DataIC50: 4.30nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 73nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 88nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 137nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 334nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 359nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 639nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 669nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 733nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 894nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 980nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+3nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.55E+5nMAssay Description:Inhibition of human plasma BuchE by Ellman's methodMore data for this Ligand-Target Pair