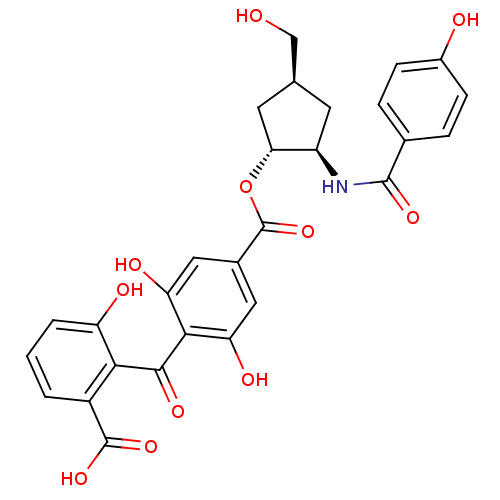
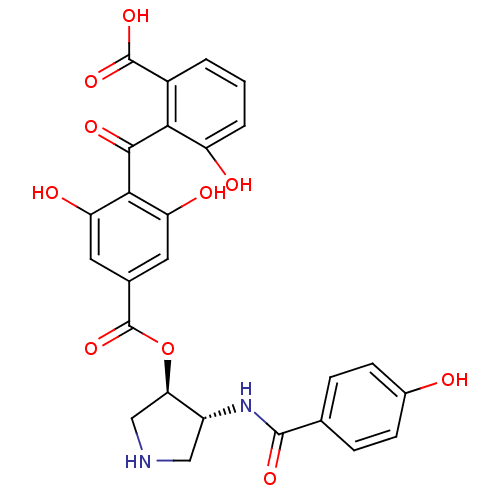
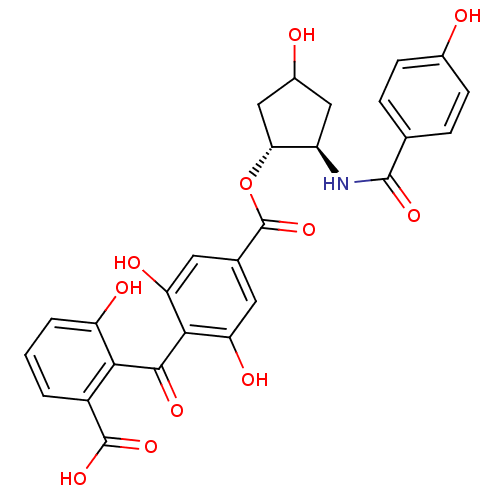
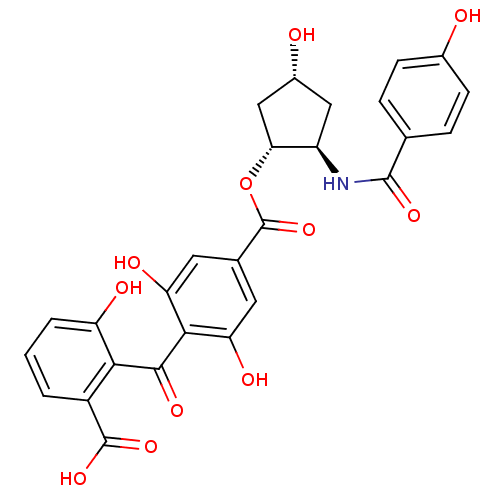
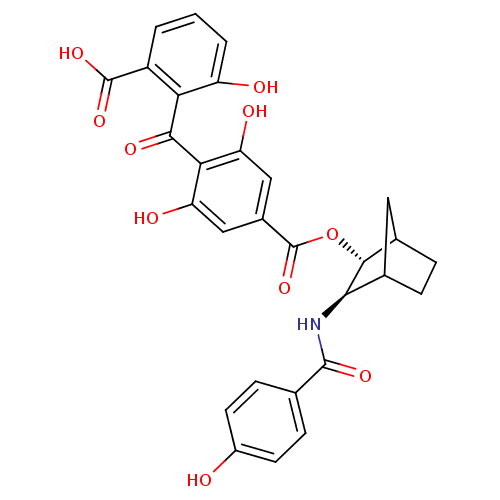
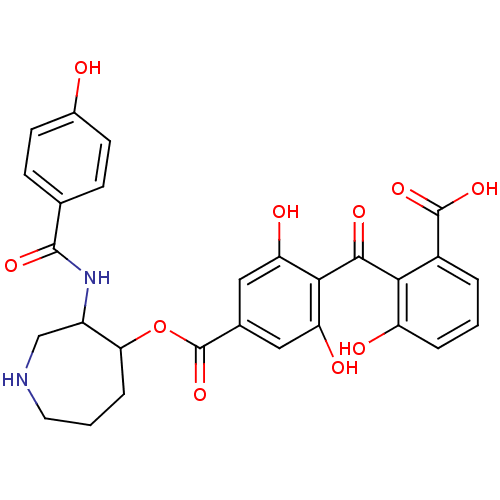
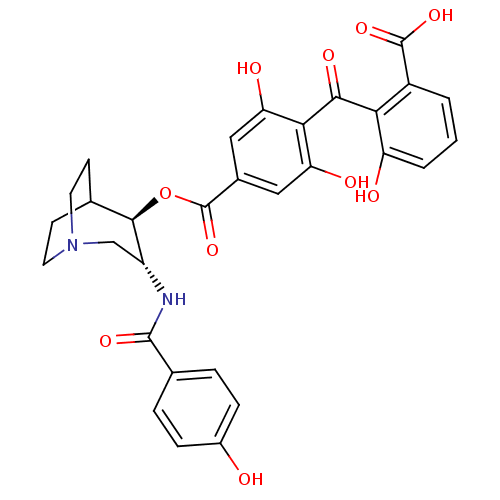
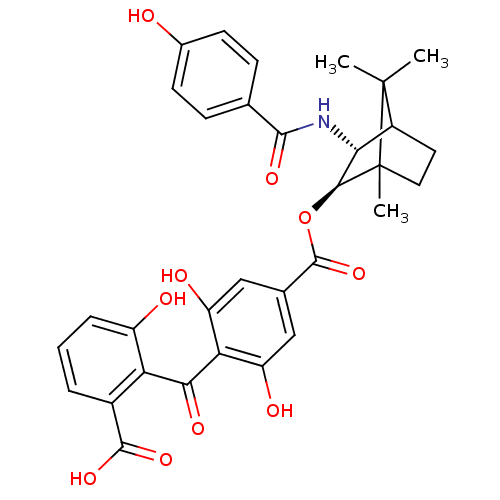
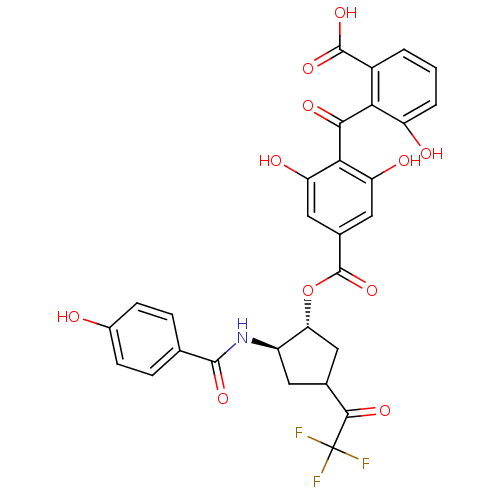
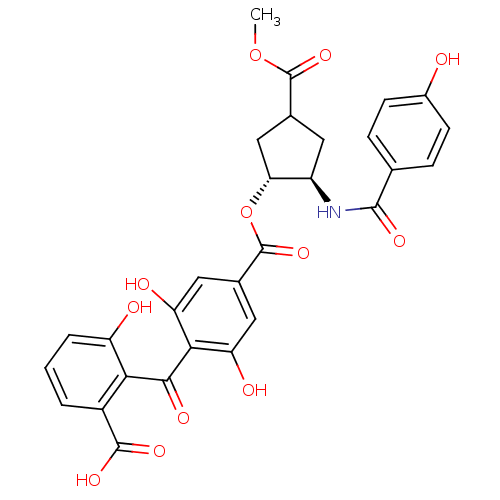
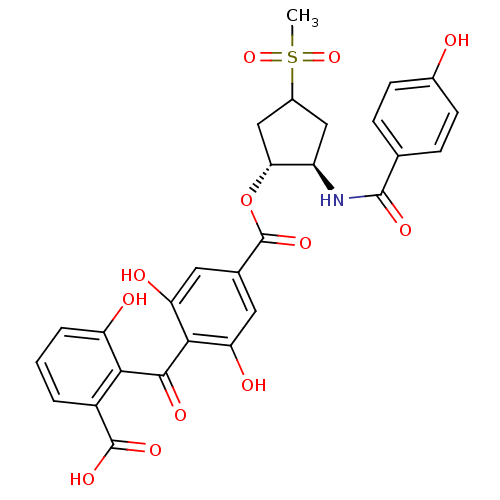
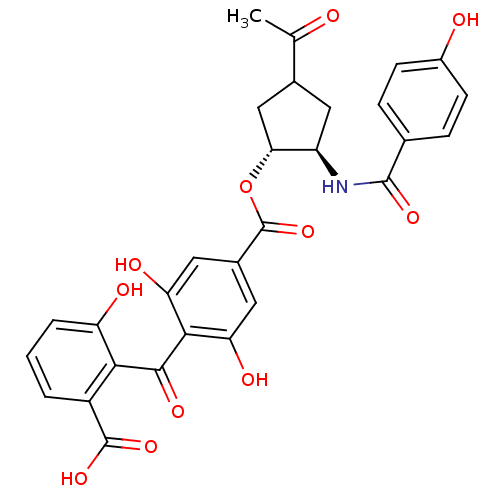
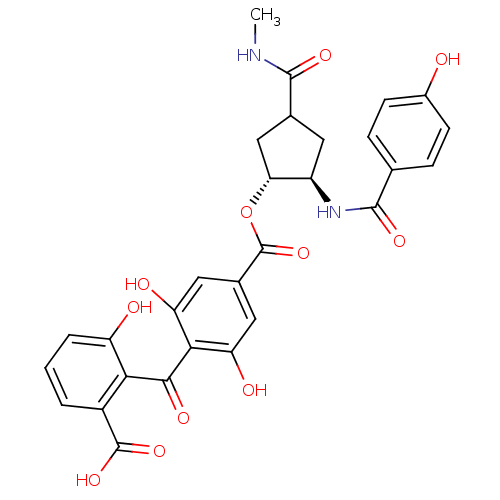
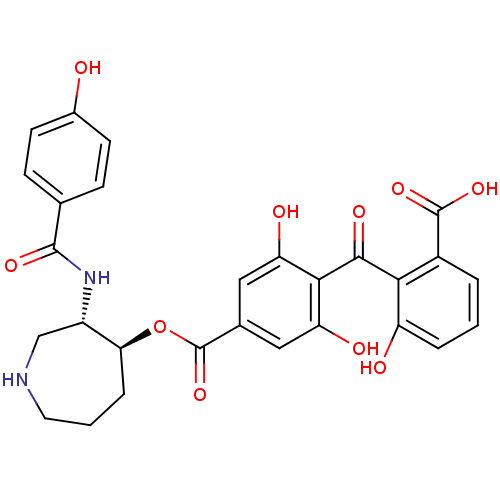
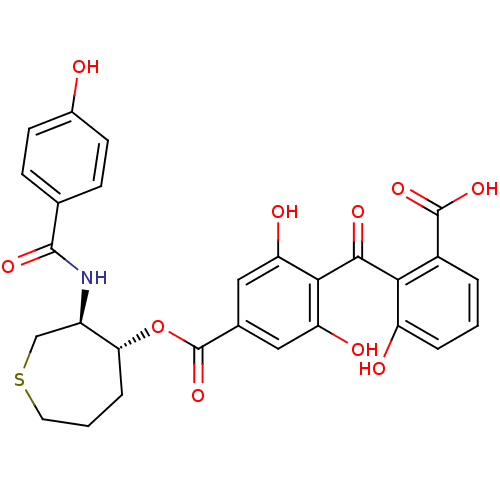
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
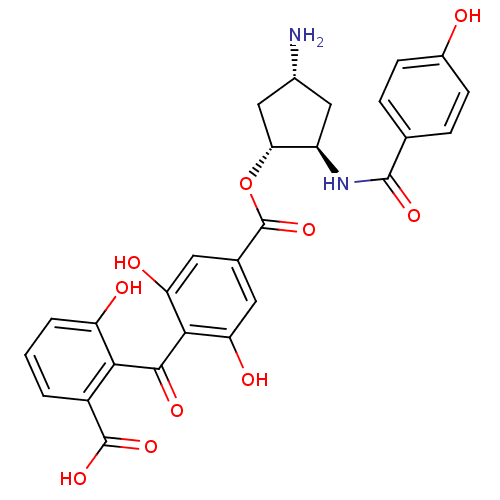
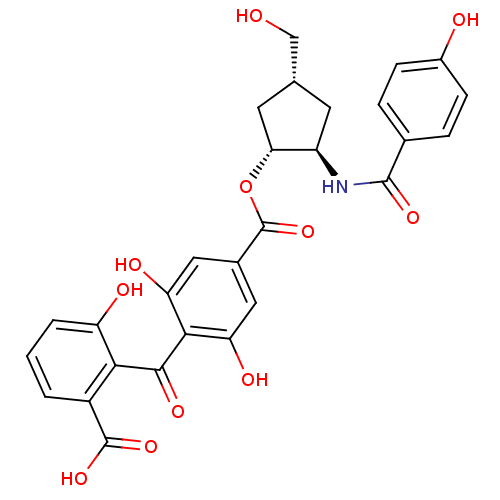
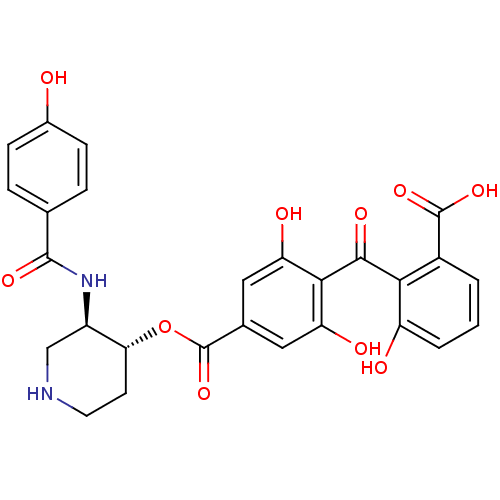
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
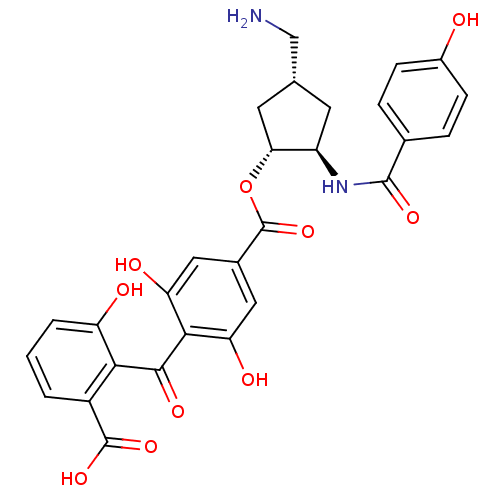
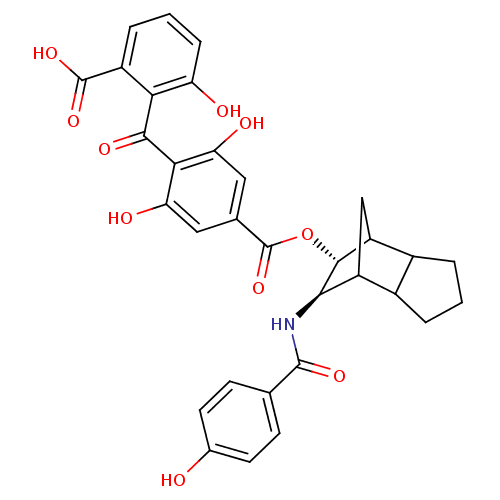
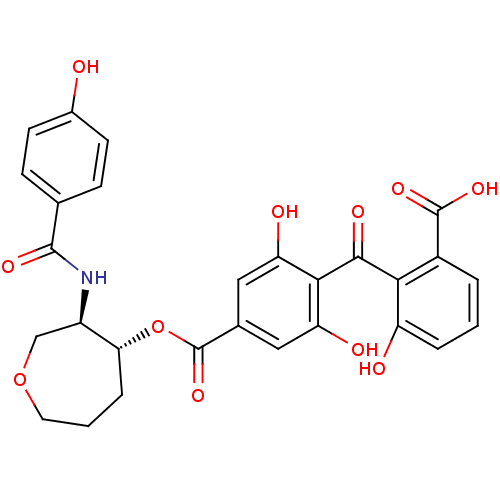
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
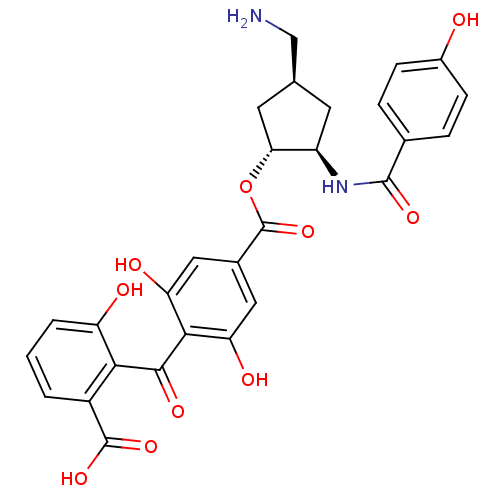
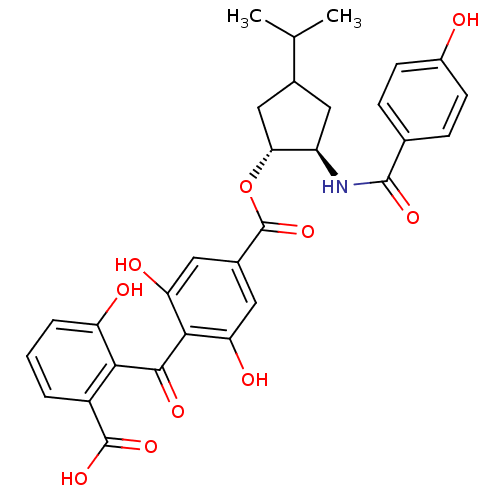
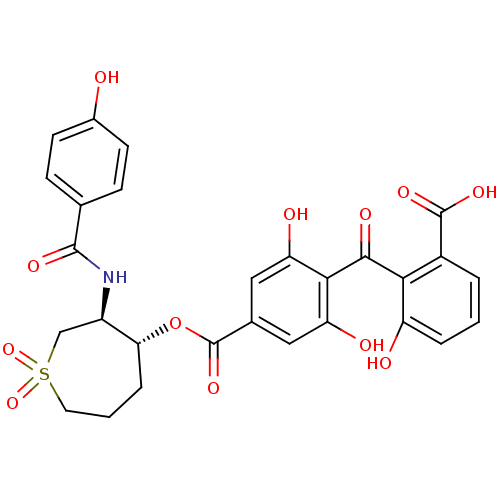
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 410nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 410nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 430nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 910nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 5.10E+3nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 6.70E+3nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 9.60E+3nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
A Division of Eli Lilly & Company
Curated by ChEMBL
A Division of Eli Lilly & Company
Curated by ChEMBL
Affinity DataIC50: 3.40E+4nMAssay Description:Inhibitory concentration against recombinant human Protein kinase C alpha isozymeMore data for this Ligand-Target Pair