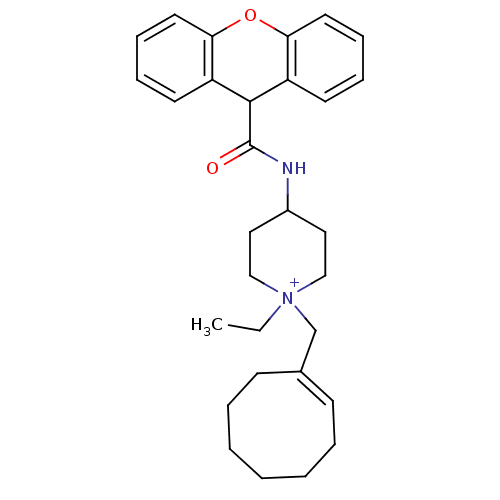
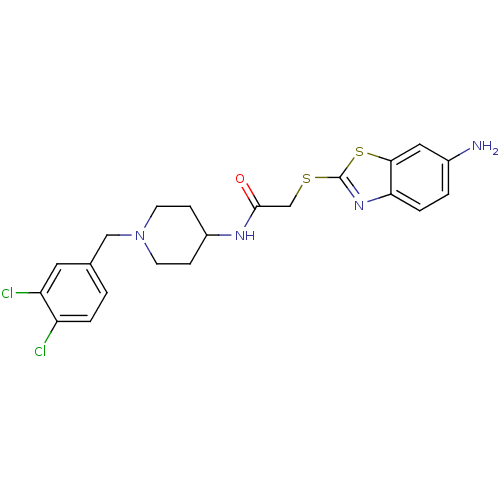
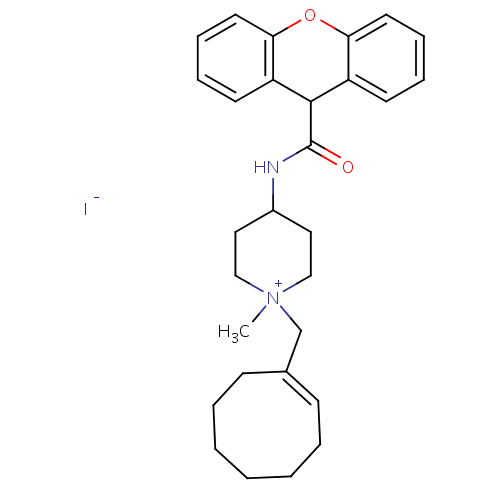
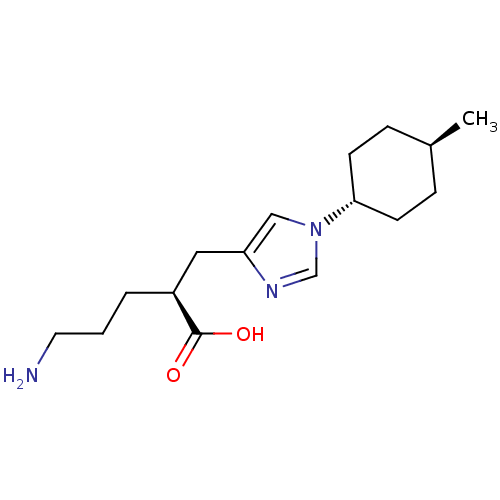
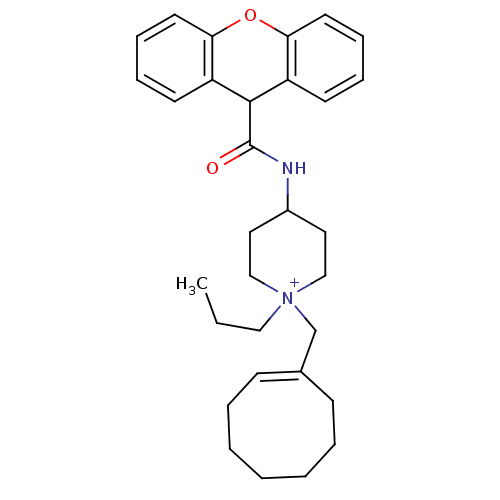
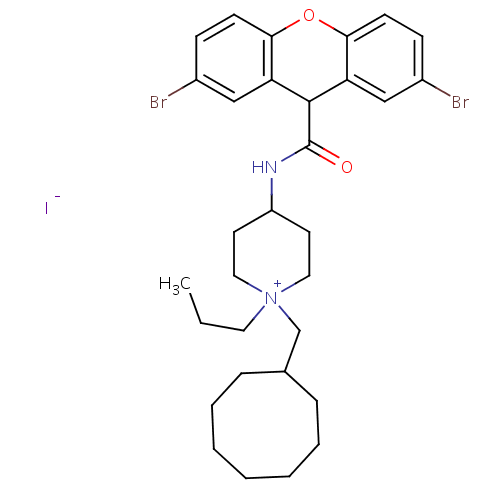
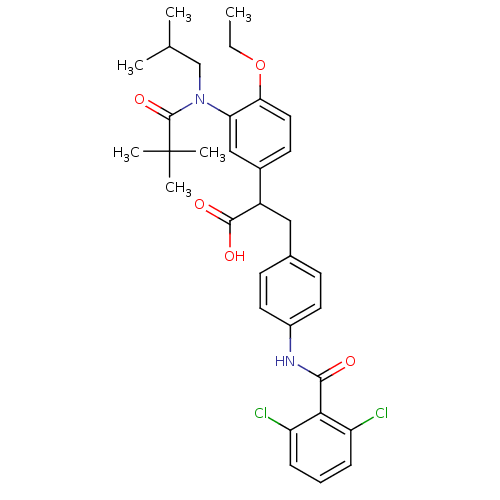
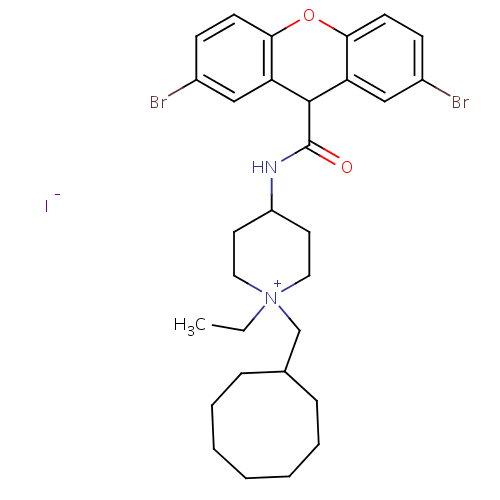
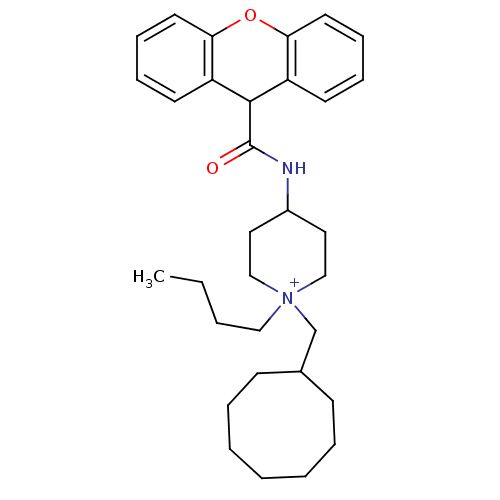
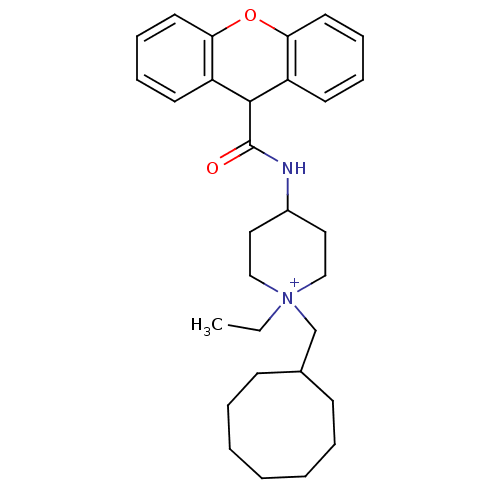
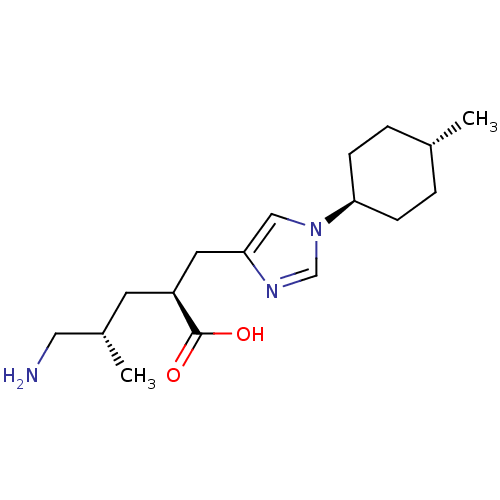
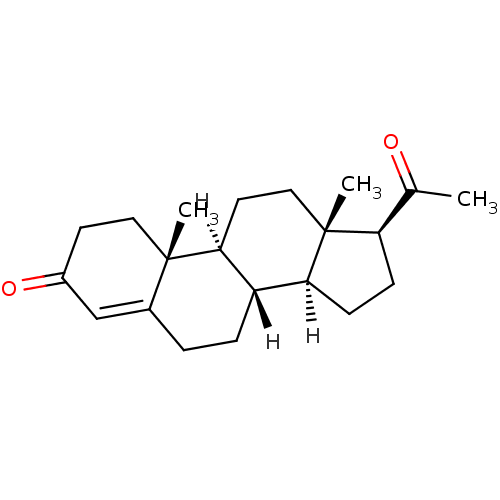
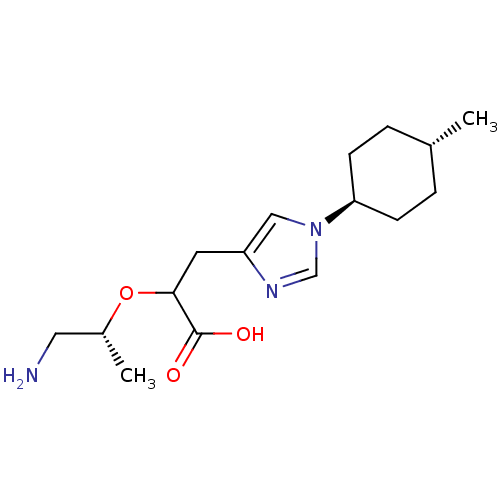
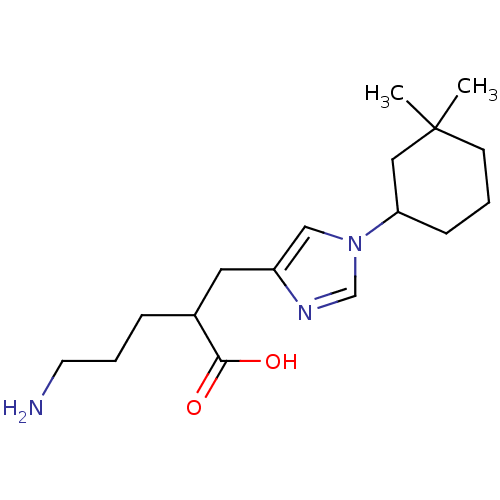
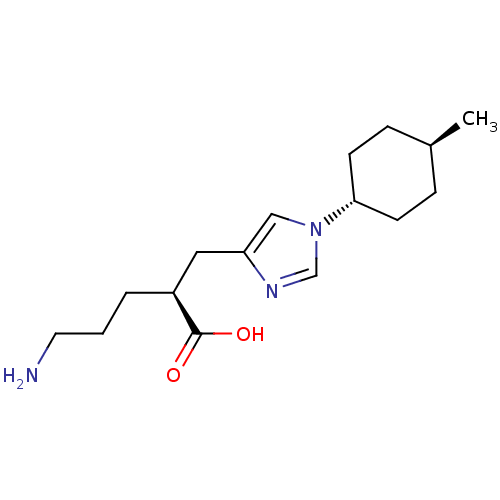
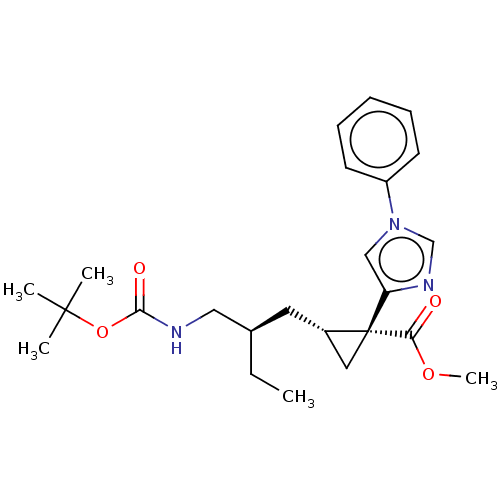
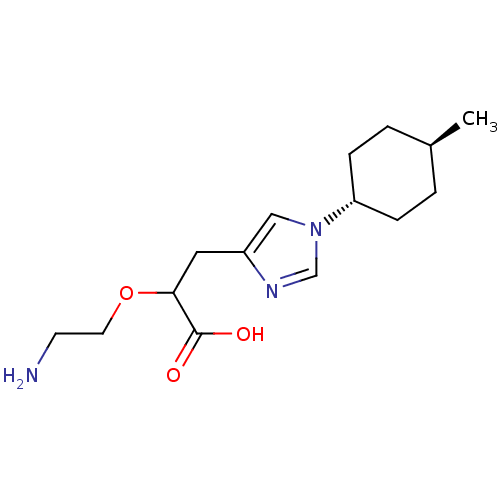
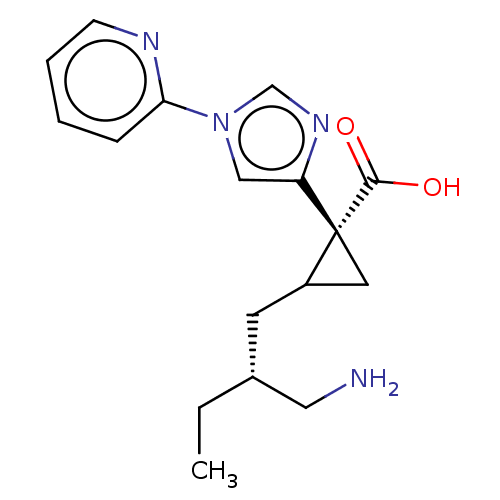
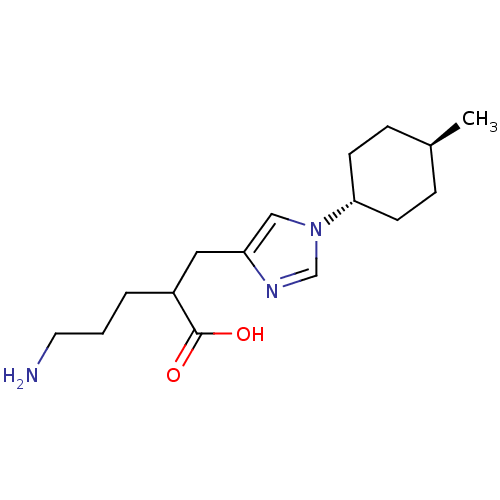
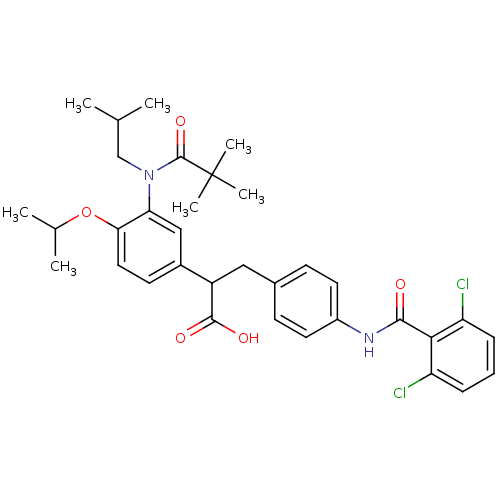
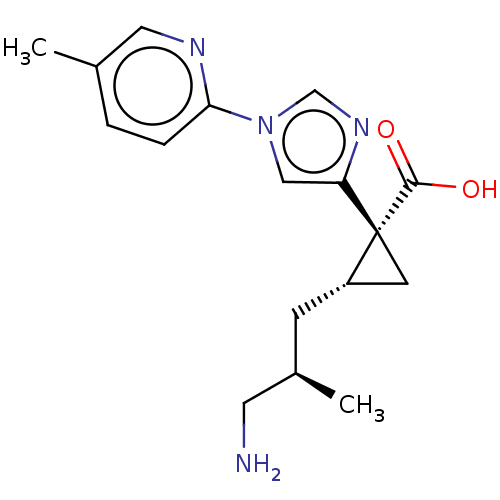
Affinity DataIC50: 0.100nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.580nMAssay Description:Inhibitory activity against I-Eotaxin binding to human CCR3 receptorsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.580nMAssay Description:Concentration required for 50% inhibition of [125I]-Eotaxin binding to human CCR3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.650nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.730nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Concentration required for 50% inhibition of [125I]-Eotaxin binding to human CCR1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Displacement of [3H]testosterone from androgen receptor in Sprague-Dawley rat prostate gland after 2 hrs by liquid scintillation countingChecked by AuthorMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Concentration required for 50% inhibition of [125I]-Eotaxin binding to human CCR3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Enzyme inhibition assay using TAFIa.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Competitive binding affinity to rat androgen receptorChecked by AuthorMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Displacement of [3H]testosterone from Sprague-Dawley rat AR by liquid scintillation countingMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:HEPES buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.4; hereinafter, referred to as HBS) was used in the preparation of a reaction solution. To 12 &...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Competitive binding affinity to rat androgen receptorChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Enzyme inhibition assay using TAFIa.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.20nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Enzyme inhibition assay using TAFIa.More data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Mus musculus)
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.80nMAssay Description:Inhibitory activity against 125 I -MIP-1 alpha binding to mouse CCR1 receptorsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 1(Mus musculus)
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.80nMAssay Description:Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Displacement of [3H]progesterone from progesterone receptor in JW rabbit uterus after 2 hrs by liquid scintillation countingChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Displacement of [3H]progesterone from rabbit PR by liquid scintillation countingMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.10nMAssay Description:Inhibitory activity against I-Eotaxin induced [Ca2+] response in human CCR3 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Enzyme inhibition assay using TAFIa.More data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Enzyme inhibition assay using TAFIa.More data for this Ligand-Target Pair
Affinity DataIC50: 7.80nMAssay Description:Enzyme inhibition assay using TAFIa.More data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:HEPES buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.4; hereinafter, referred to as HBS) was used in the preparation of a reaction solution. To 12 &...More data for this Ligand-Target Pair
Affinity DataIC50: 8.10nMAssay Description:Enzyme inhibition assay using TAFIa.More data for this Ligand-Target Pair
Affinity DataIC50: 8.30nMAssay Description:HEPES buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.4; hereinafter, referred to as HBS) was used in the preparation of a reaction solution. To 12 &...More data for this Ligand-Target Pair
Affinity DataIC50: 8.30nMAssay Description:Enzyme inhibition assay using TAFIa.More data for this Ligand-Target Pair
Affinity DataIC50: 8.40nMAssay Description:Inhibition of Very late antigen-4 (VLA-4) expressing human leukemia cells (HL-60) aggregation with human Vascular cell adhesion molecule-1 (VCAM-1) e...More data for this Ligand-Target Pair
Affinity DataIC50: 8.70nMAssay Description:HEPES buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.4; hereinafter, referred to as HBS) was used in the preparation of a reaction solution. To 12 &...More data for this Ligand-Target Pair
Affinity DataIC50: 8.70nMAssay Description:HEPES buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.4; hereinafter, referred to as HBS) was used in the preparation of a reaction solution. To 12 &...More data for this Ligand-Target Pair


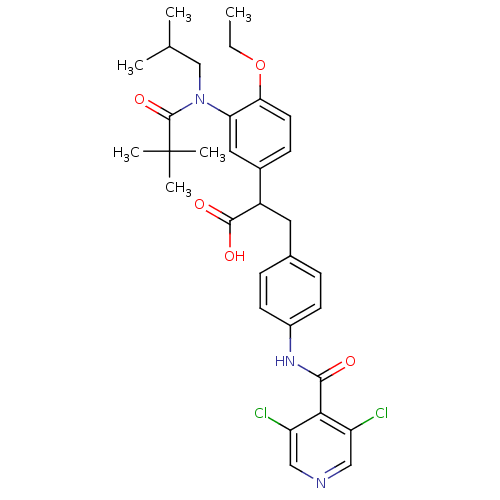
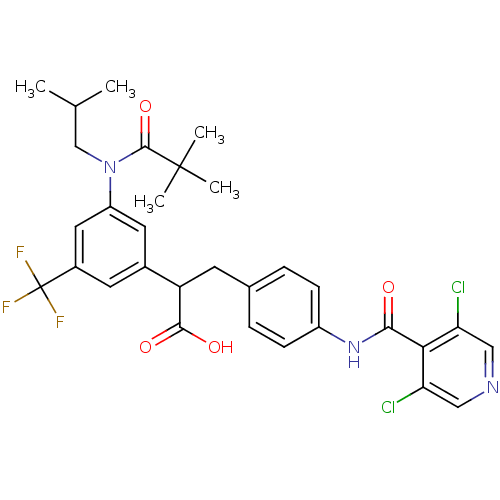

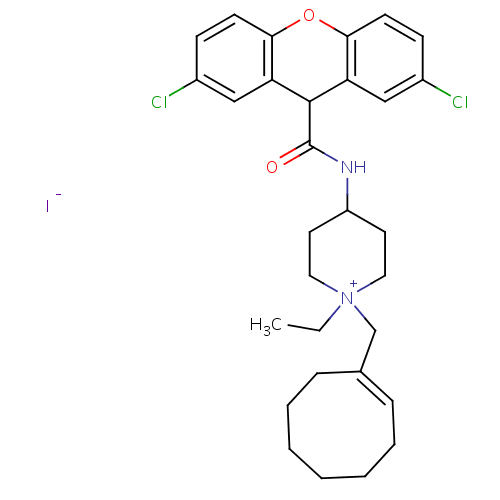
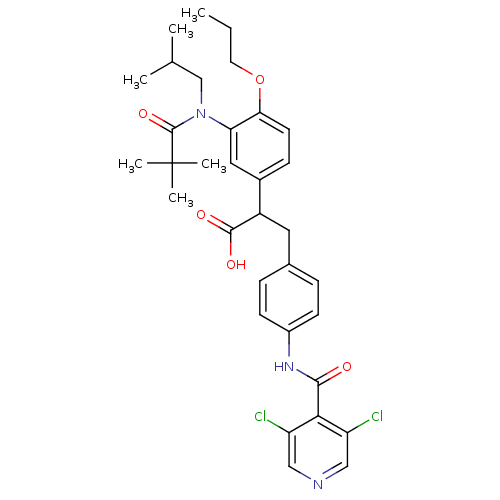
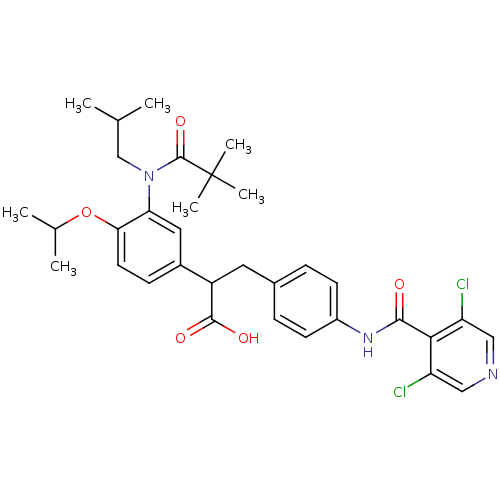
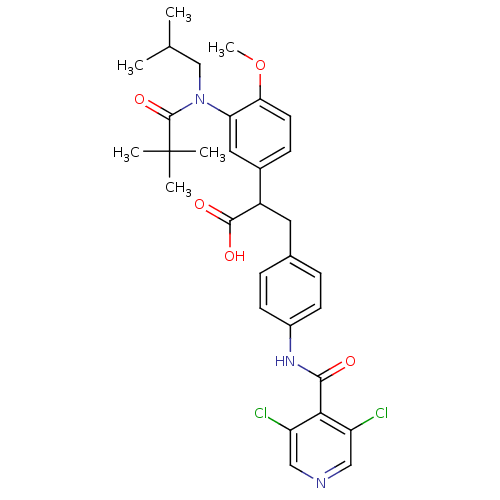
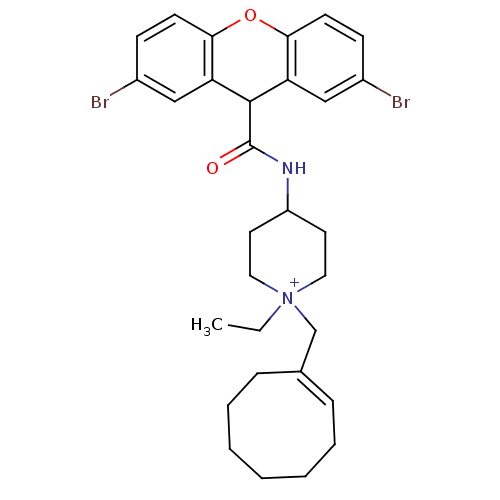
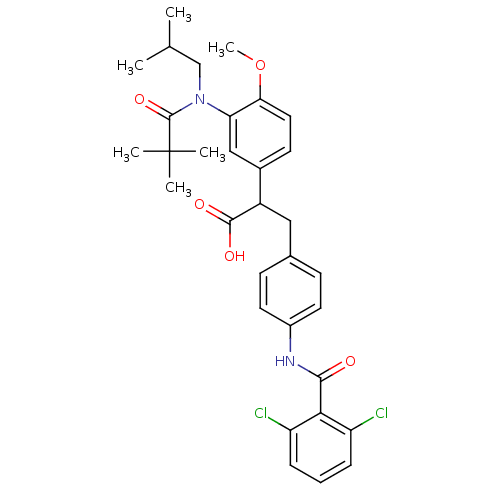

 3D Structure (crystal)
3D Structure (crystal)