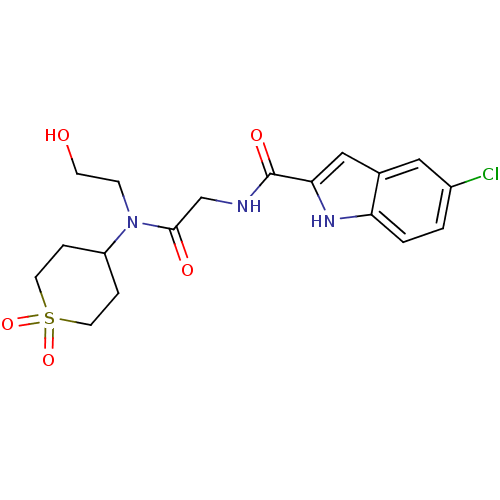
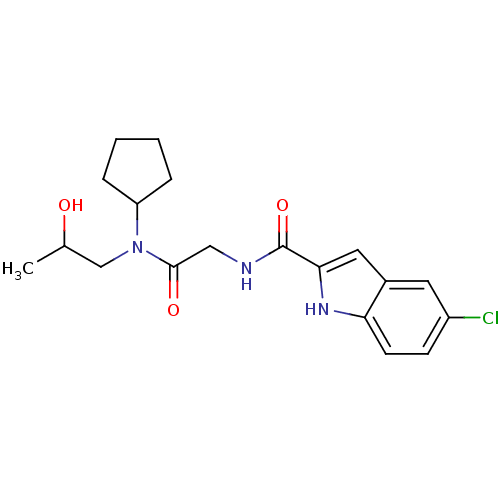
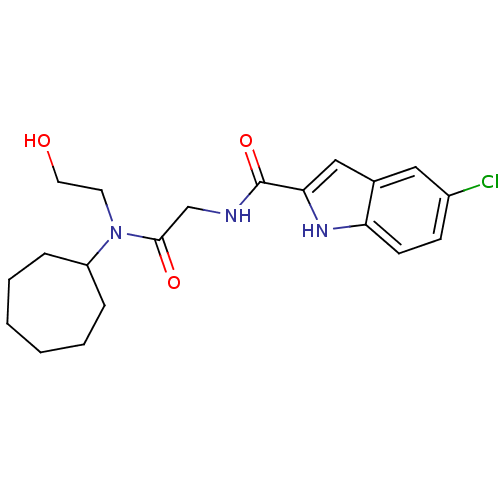
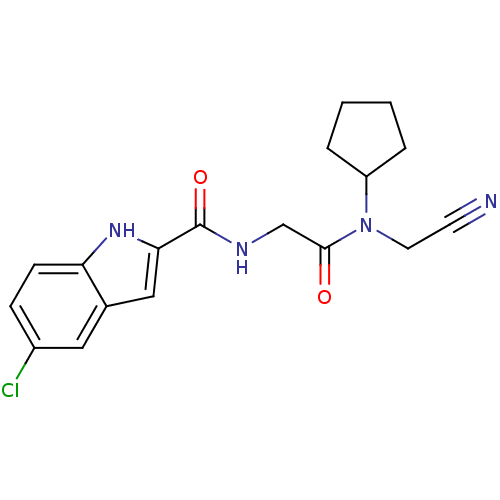
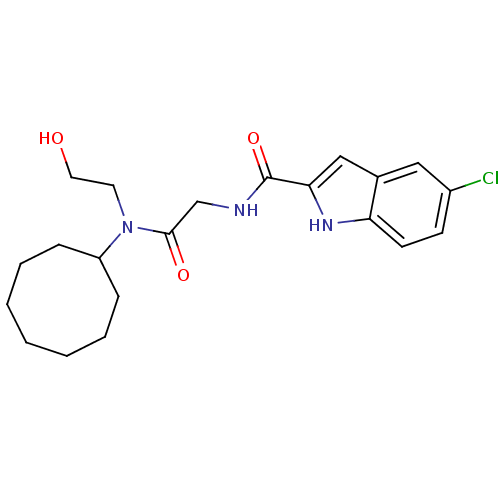
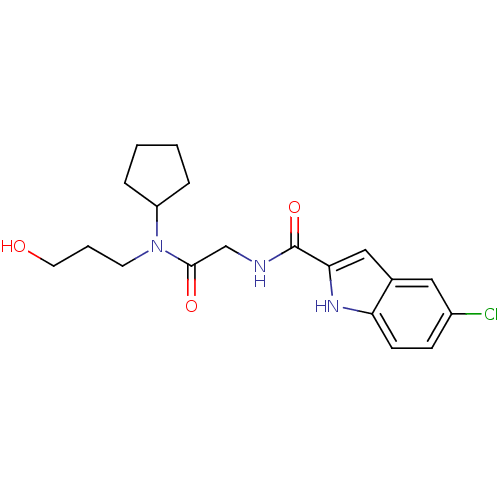
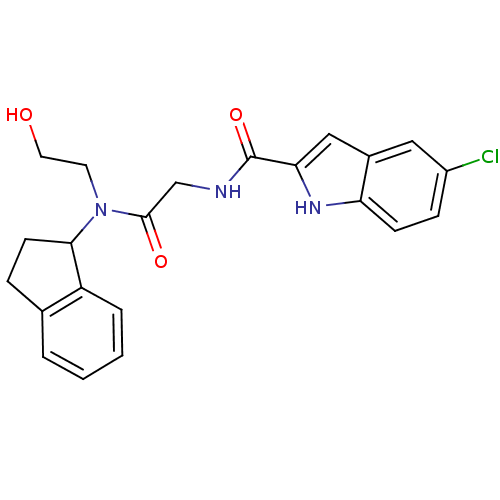
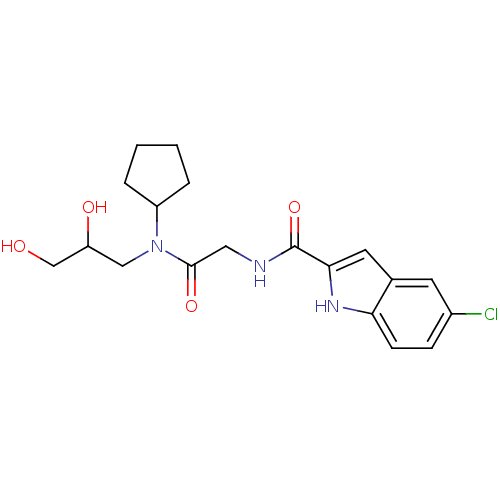
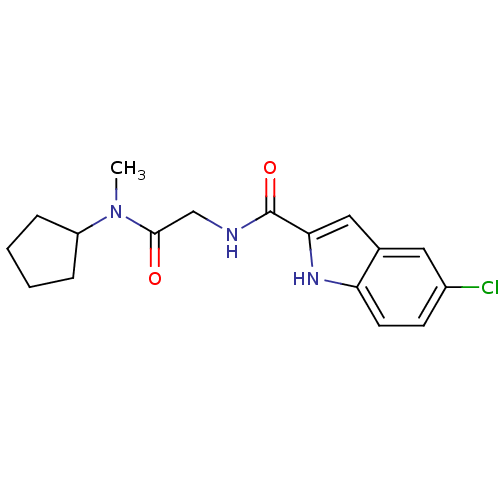
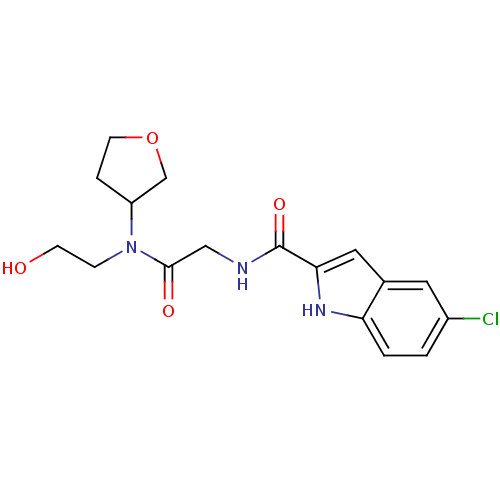
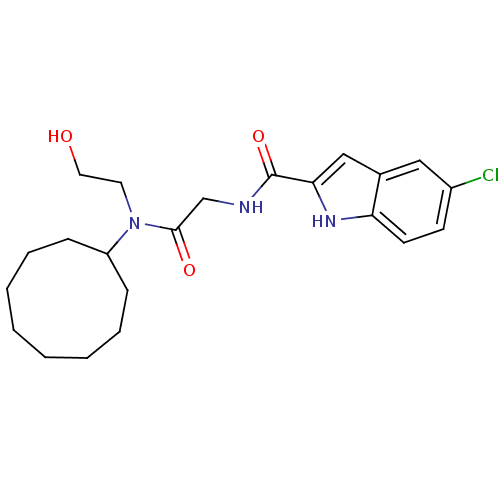
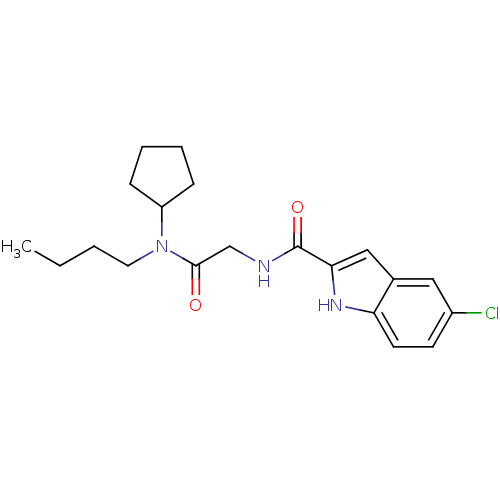
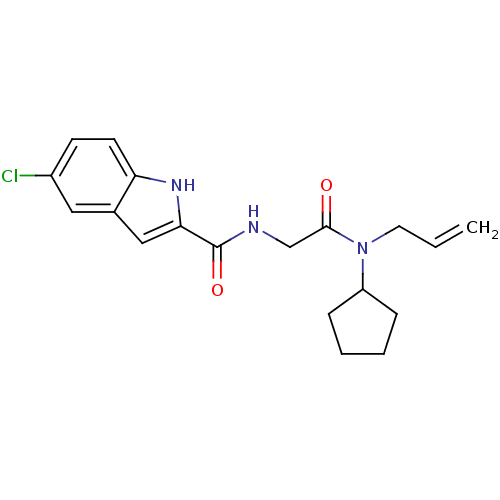
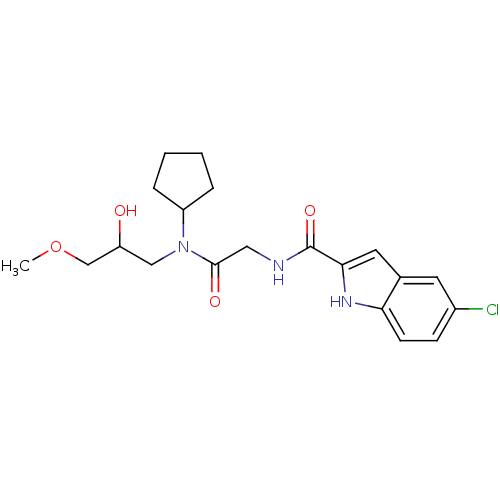
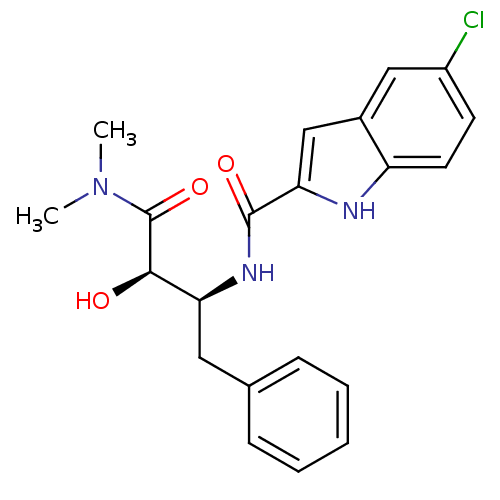
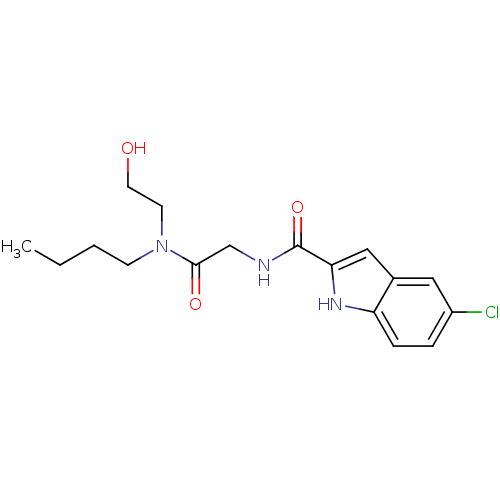
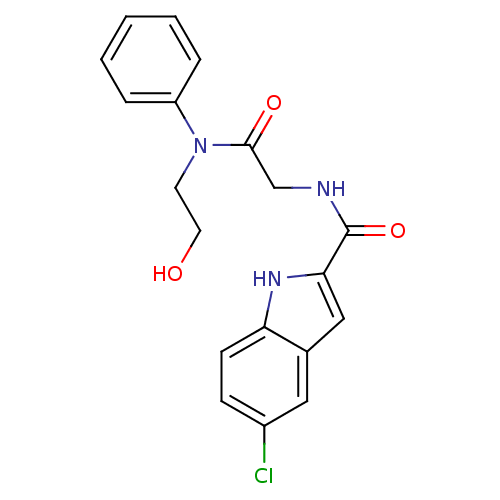
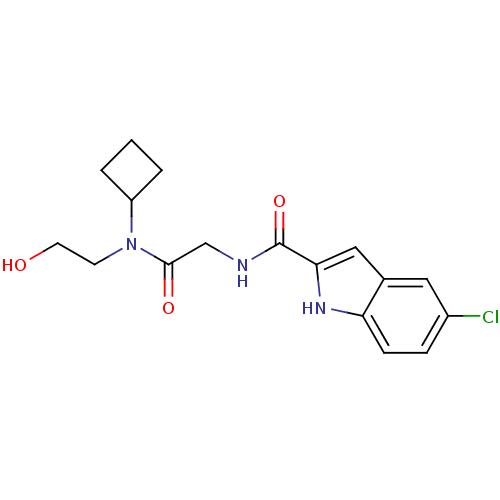
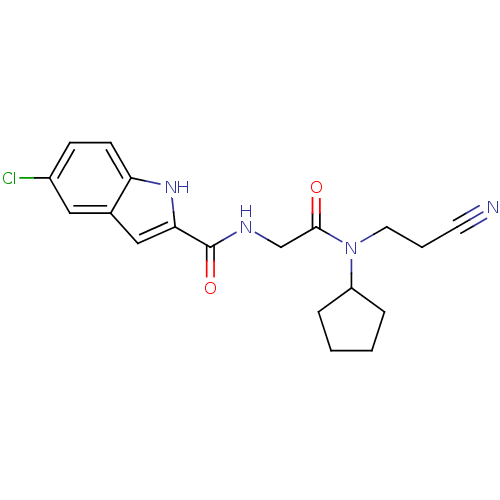
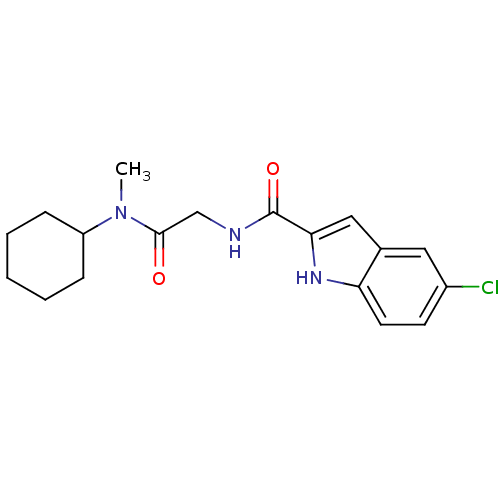
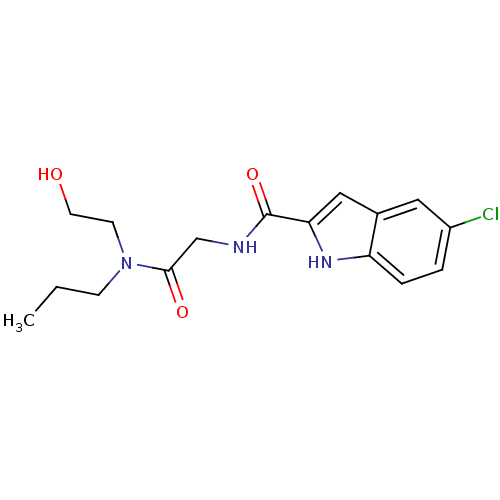
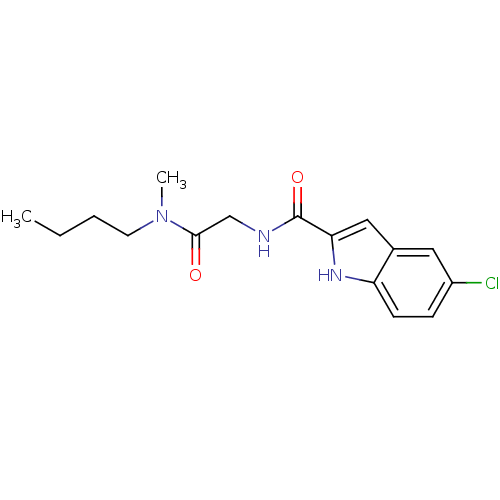
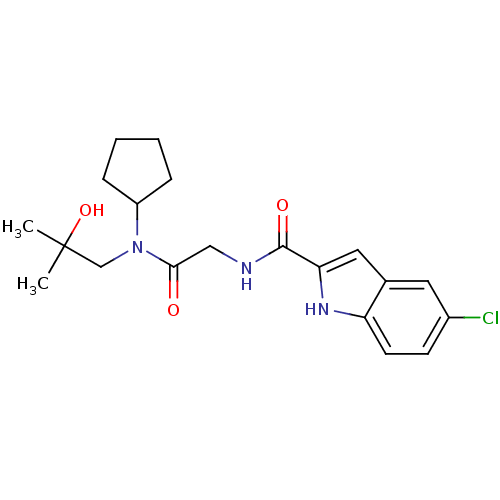
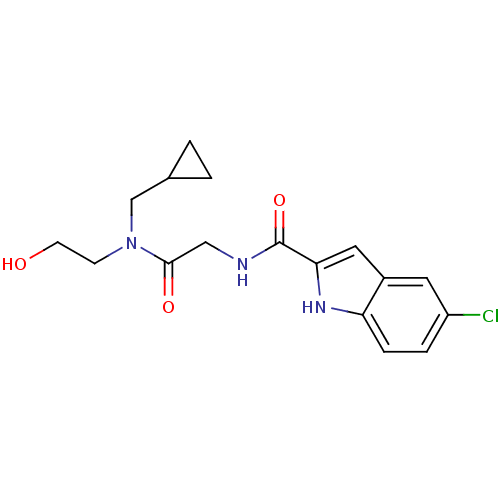
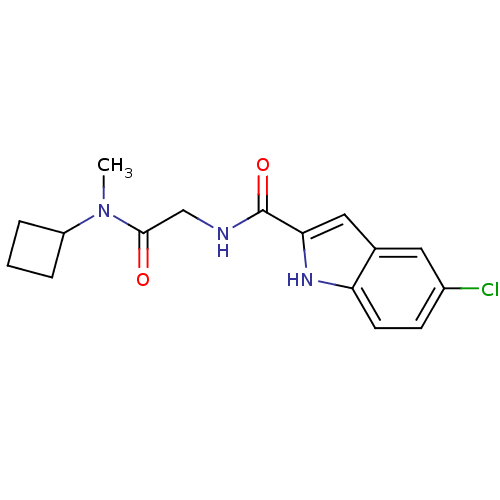
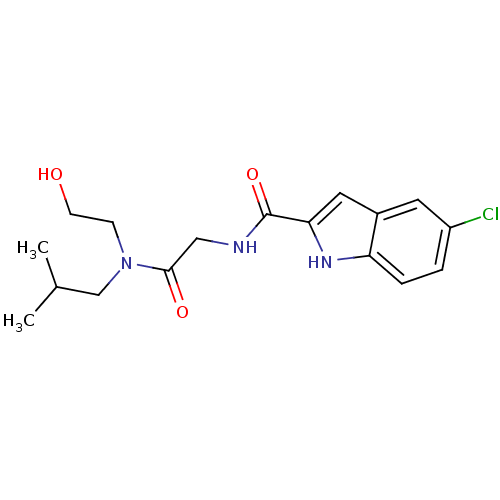
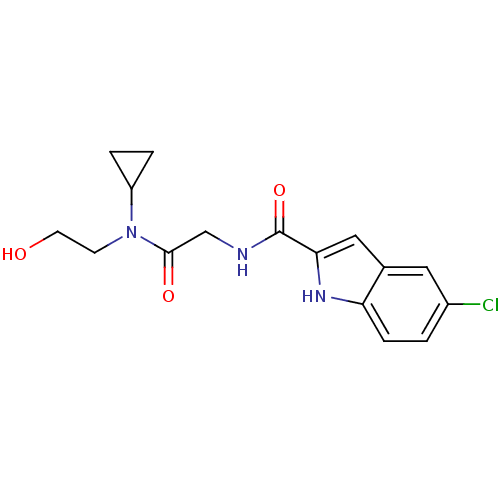
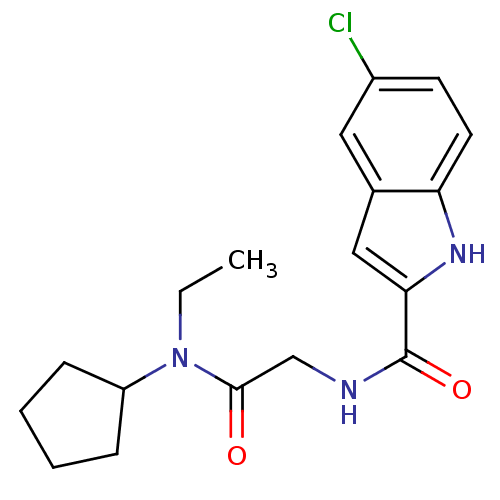
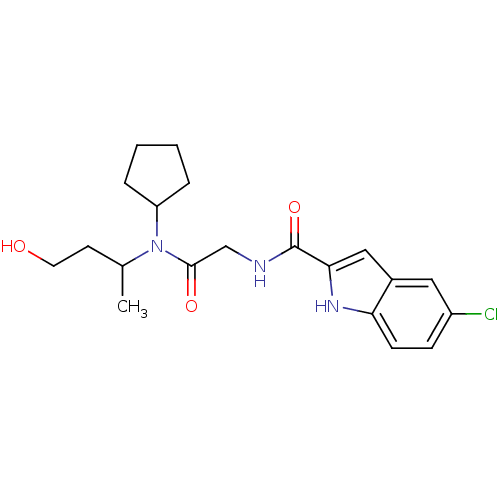
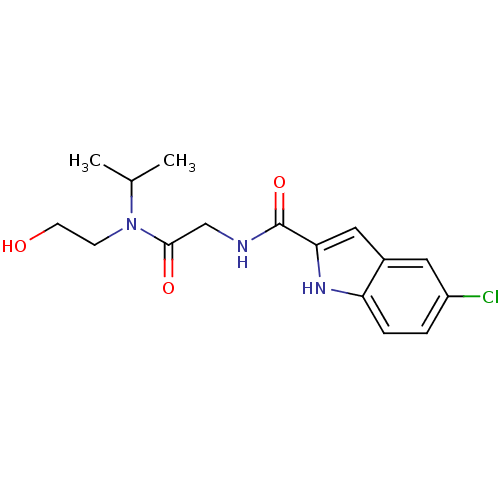
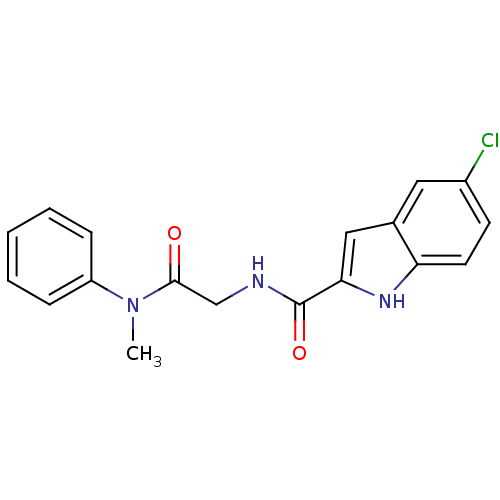
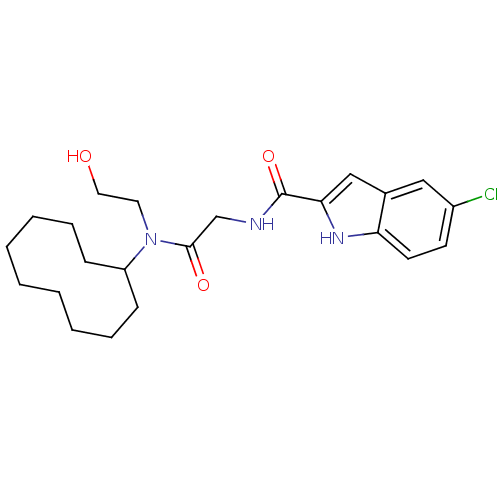
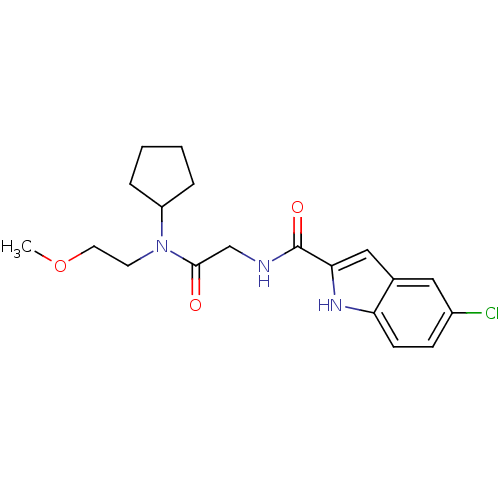
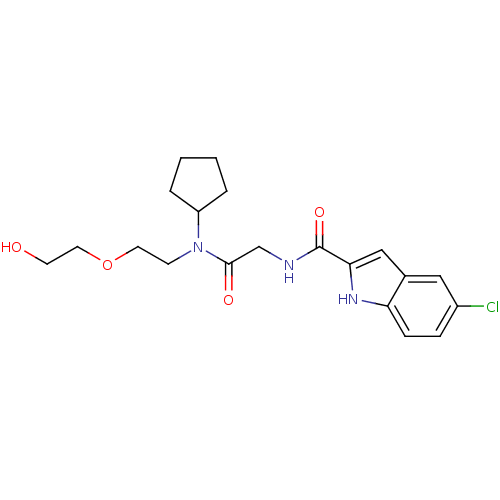
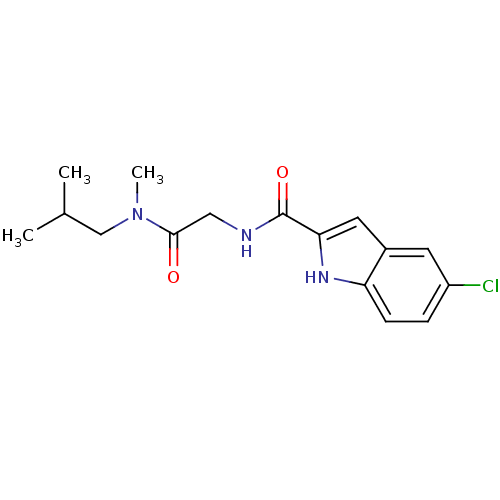
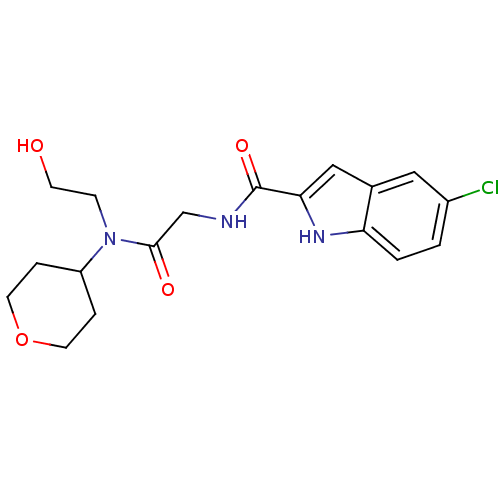
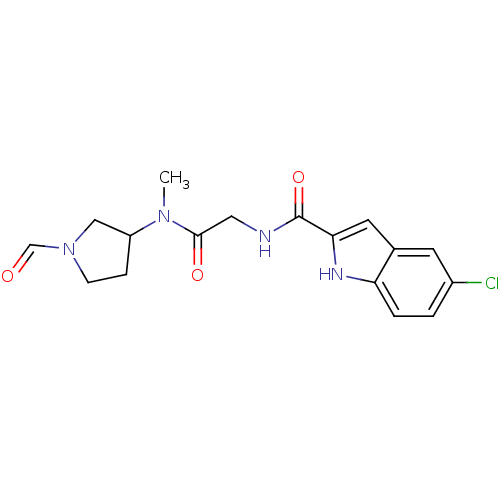
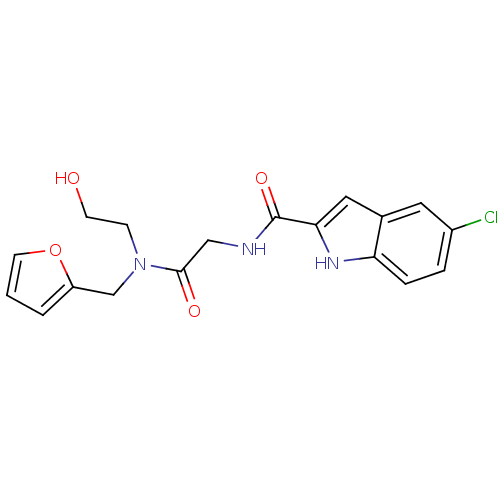
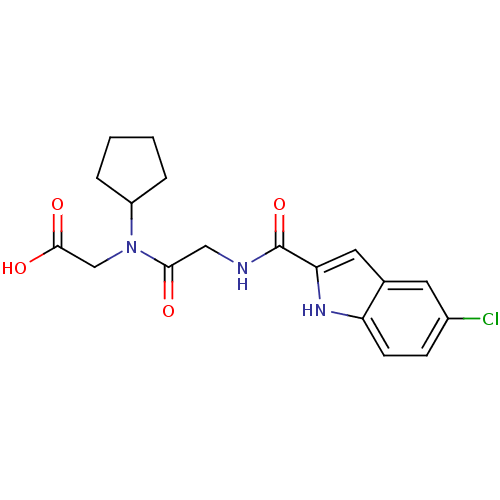
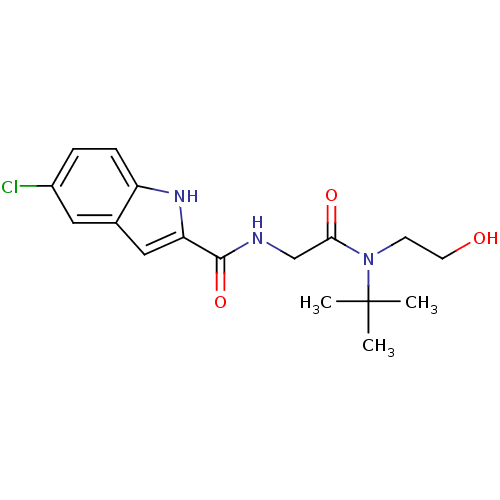
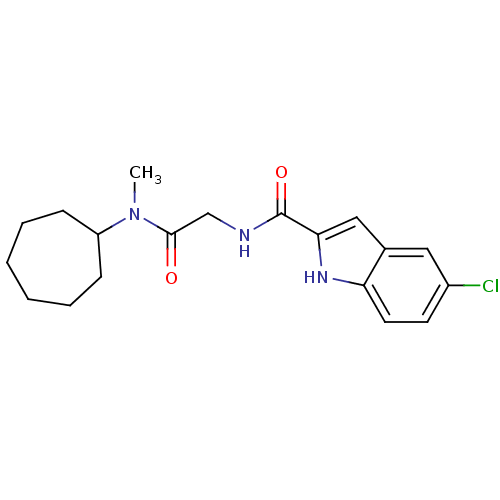
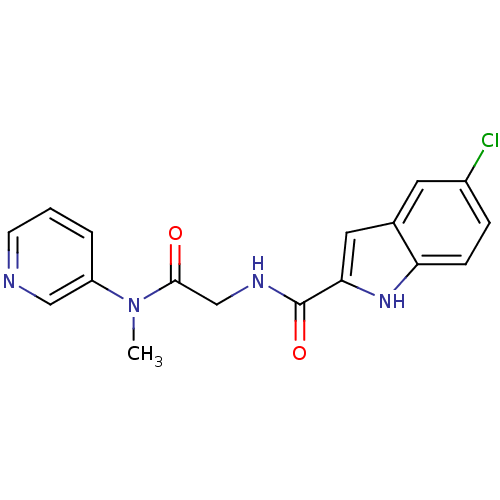
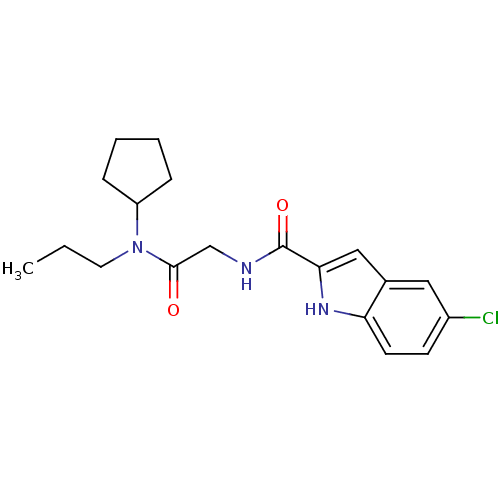
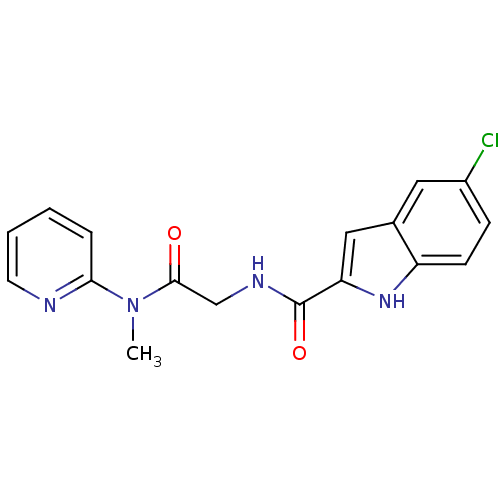
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 49nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 54nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 83nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 96nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 99nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 260nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair
TargetGlycogen phosphorylase, liver form(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 370nMAssay Description:Inhibitory concentration against glycogen phosphorylaseMore data for this Ligand-Target Pair