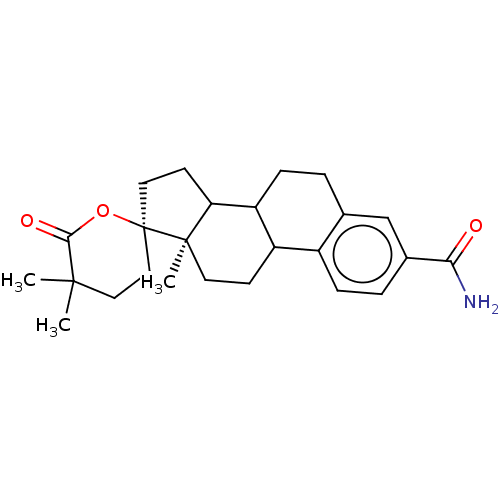
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 6.90nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
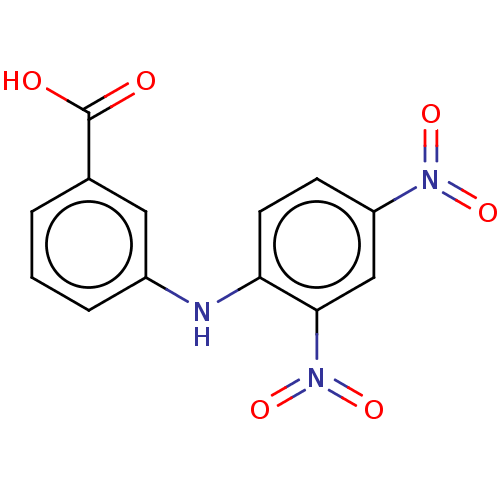
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 380nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
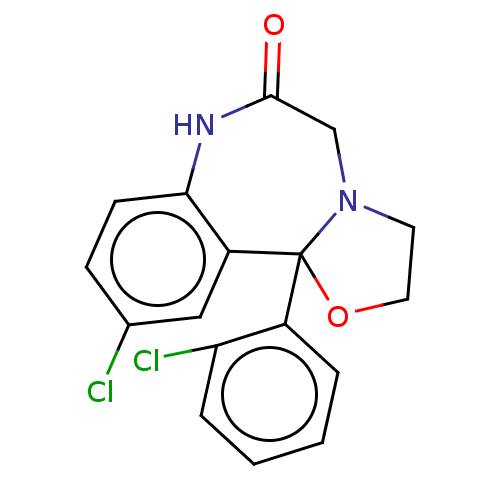
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.32E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.50E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.66E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 6.00E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 8.20E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.50E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 2.10E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
The Trustees of the University of Pennsylvania
US Patent
The Trustees of the University of Pennsylvania
US Patent
Affinity DataKi: 1.06E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)