TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of cyclin dependent kinase 1-cyclinBMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human cdk5/p35More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human cdk2/cyclin AMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human cdc2/cyclin B assessed as [32P] incorporation in histone H1 from [gamma32P]ATPMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of Cyclin A-cyclin-dependent kinase 2More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B(Marthasterias glacialis (starfish))
Universidad San Pablo CEU
Universidad San Pablo CEU
Affinity DataIC50: 6nMT: 2°CAssay Description:Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-33P] ATP. 33...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2(Homo sapiens (Human))
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of Cyclin E-cyclin-dependent kinase 2More data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibition of human cdk2/cyclin EMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 50nMAssay Description:Inhibition of starfish cdc2/cyclin B assessed as [32P] incorporation in histone H1 from 150 M [gamma32P]ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Inhibition of starfish cdc2/cyclin B assessed as [32P] incorporation in histone H1 from 1.5 mM [gamma32P]ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of Saccharomyces cerevisiae cdc28More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of human insulin receptorMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 3(Homo sapiens (Human))
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of Mitogen-activated protein kinase 3 (MAPK-ERK1)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 3(Homo sapiens (Human))
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Affinity DataIC50: 3.33E+3nMAssay Description:Inhibition of human Erk1More data for this Ligand-Target Pair
TargetcAMP-dependent protein kinase catalytic subunit alpha/beta/gamma(Homo sapiens (Human))
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of cAMP-dependent protein kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human cdk4/cyclin D1More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of human GSK3-betaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D1(Homo sapiens (Human))
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Cyclin D1-cyclin-dependent kinase 4More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Binding affinity to cyclin-dependent kinase 1 (CDK1)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human PKCetaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human PKCzetaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human PKCepsilonMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human PKCdeltaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human PKCgammaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human PKCbeta1More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human PKCalphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human PKCbeta2More data for this Ligand-Target Pair

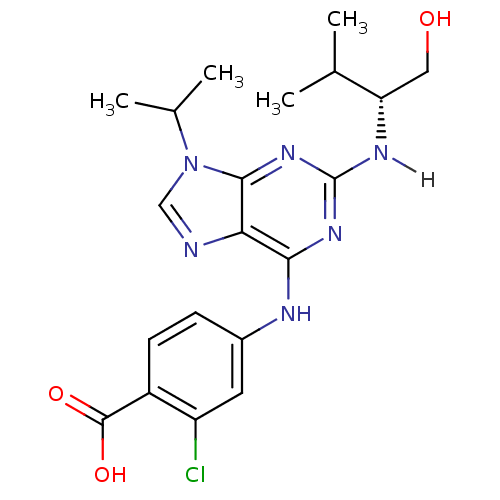
 3D Structure (crystal)
3D Structure (crystal)