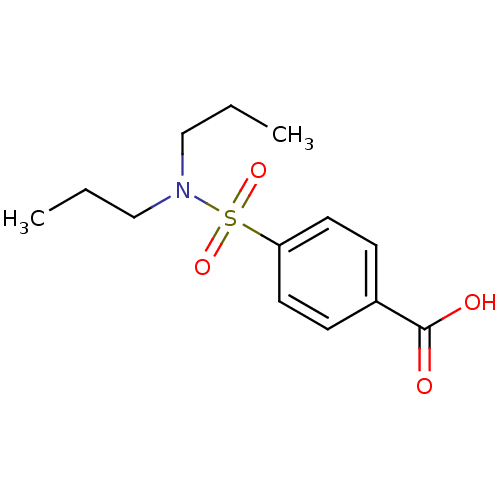
TargetUDP-glucuronosyltransferase 1A1(Homo sapiens (Human))
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Toyama Chemical Co., Ltd.
Curated by ChEMBL
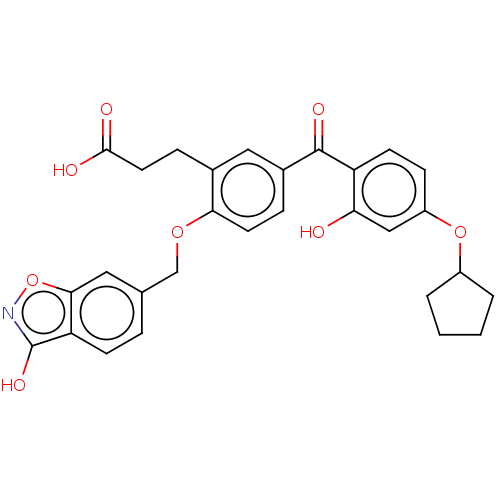
Affinity DataKi: 1.54E+3nMAssay Description:Substrate inhibition of human UGT1A1-mediated T-5224 acyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC meth...More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1A1(Homo sapiens (Human))
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: 4.67E+3nMAssay Description:Substrate inhibition of human UGT1A1-mediated T-5224 hydroxyl O-glucuronide formation after 10 to 60 mins in presence of UDP-glucuronic acid by HPLC ...More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1A1(Homo sapiens (Human))
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Toyama Chemical Co., Ltd.
Curated by ChEMBL
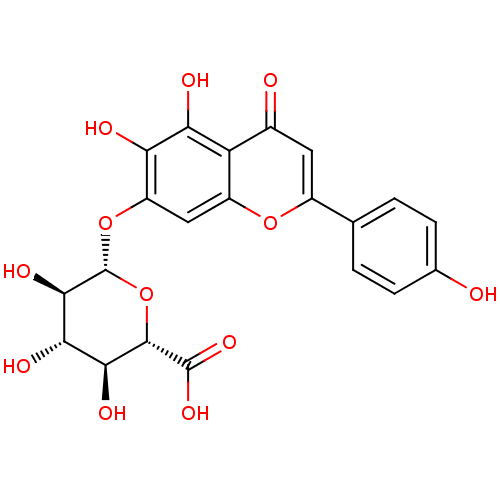
Affinity DataKi: 8.50E+3nMAssay Description:Substrate inhibition of human recombinant UGT1A1 assessed as IRI-O-5-monoglucuronide formation incubated for 5 mins prior to UDPGA addition measured ...More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1A1(Homo sapiens (Human))
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: 1.41E+4nMAssay Description:Substrate inhibition of human recombinant UGT1A1 assessed as IRI-O-4'-monoglucuronide formation incubated for 5 mins prior to UDPGA addition measured...More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1A1(Homo sapiens (Human))
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Toyama Chemical Co., Ltd.
Curated by ChEMBL
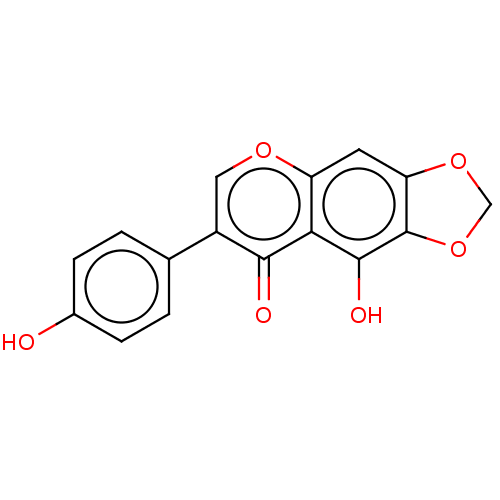
Affinity DataKi: 4.13E+4nMAssay Description:Drug metabolism assessed as human recombinant UGT1A1-mediated formation of scutellarein-7-O-glucuronide after 25 mins by HPLC/UV analysisMore data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1A1(Homo sapiens (Human))
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: 5.20E+4nMAssay Description:Inhibition of 4-methylumbelliferone glucuronidation by human recombinant UGT1A1More data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1A1(Homo sapiens (Human))
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: 9.66E+4nMAssay Description:Drug metabolism assessed as human recombinant UGT1A1-mediated formation of scutellarein-6,7-diglucuronide after 25 mins by HPLC/UV analysisMore data for this Ligand-Target Pair
TargetUDP-glucuronosyltransferase 1A1(Homo sapiens (Human))
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Toyama Chemical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: 2.21E+5nMAssay Description:Inhibition of 4-methylumbelliferone glucuronidation by human recombinant UGT1A1More data for this Ligand-Target Pair