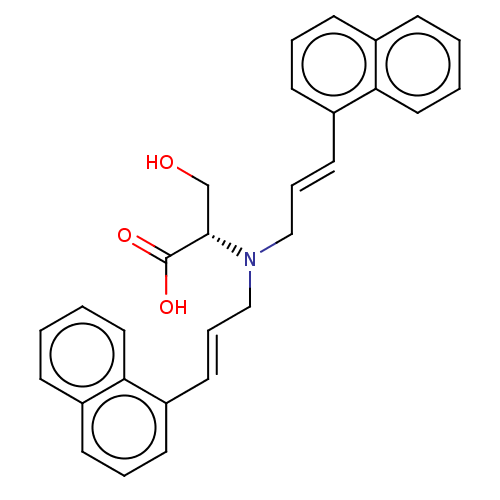
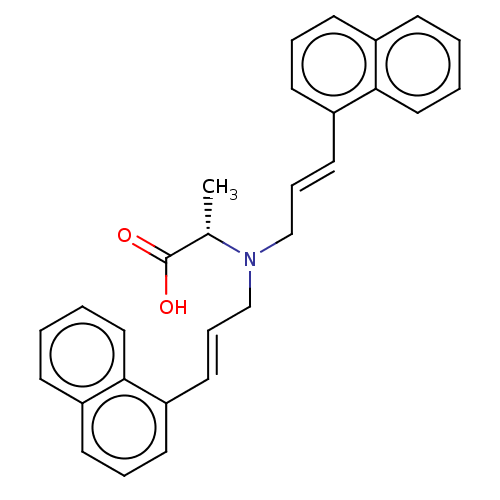
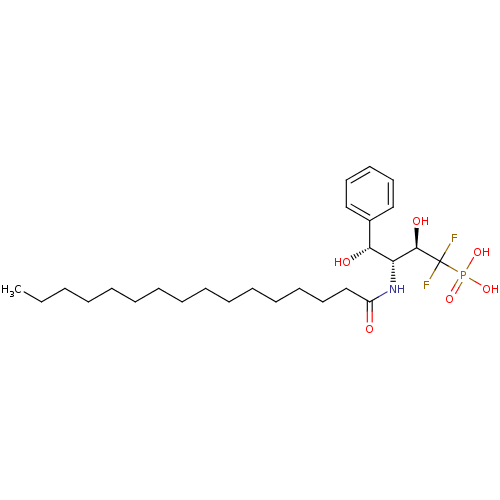
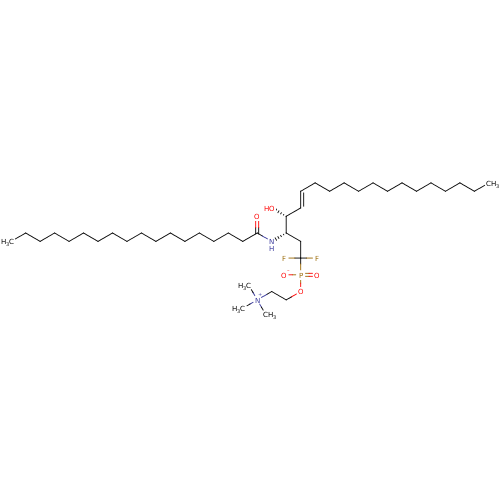
Affinity DataIC50: 300nMAssay Description:Functional potency against Tachykinin receptor 2 (NK2) in rabbit pulmonary artery assayMore data for this Ligand-Target Pair
In DepthDetails
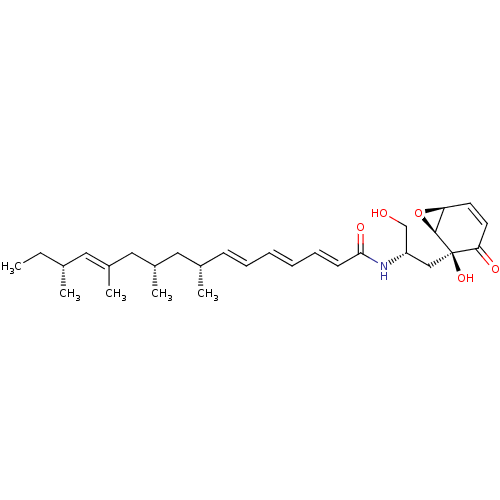
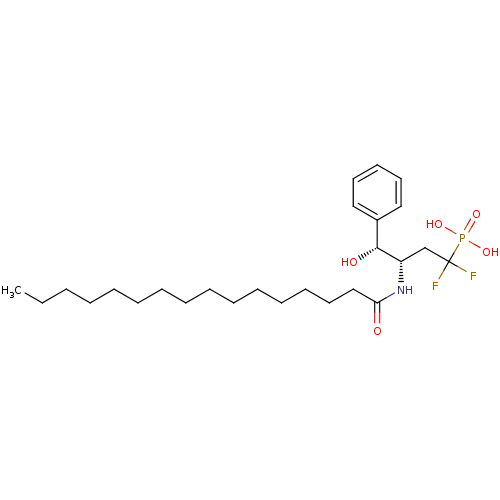
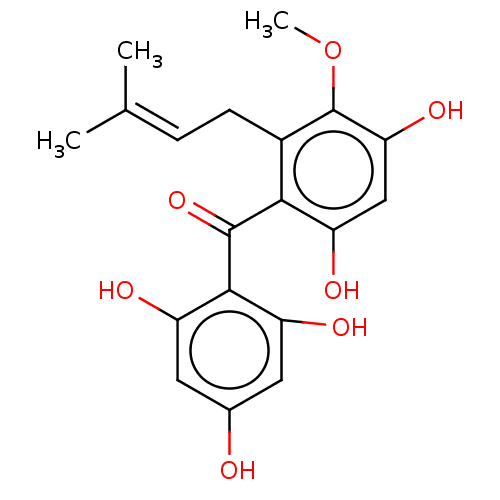
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Functional potency against Tachykinin receptor 2 (NK2) in rabbit pulmonary artery assayMore data for this Ligand-Target Pair
In DepthDetails
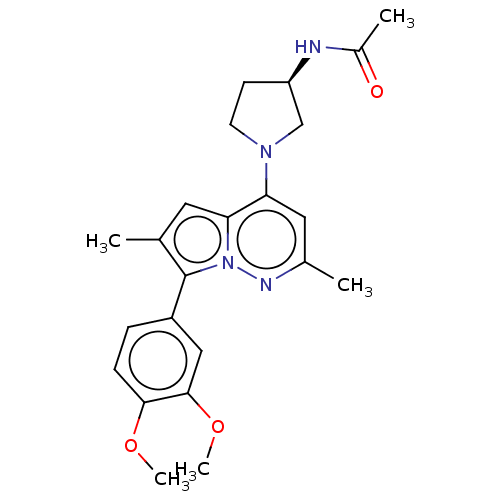
Affinity DataIC50: 1.80E+3nMAssay Description:Negative log concentration of antagonist was determined on 5-hydroxytryptamine 2B receptor of Rat stomach fundusMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 2.80E+3nMAssay Description:Negative log concentration of antagonist on 5-hydroxytryptamine 2A receptor in rat thoracic aortaMore data for this Ligand-Target Pair
In DepthDetails
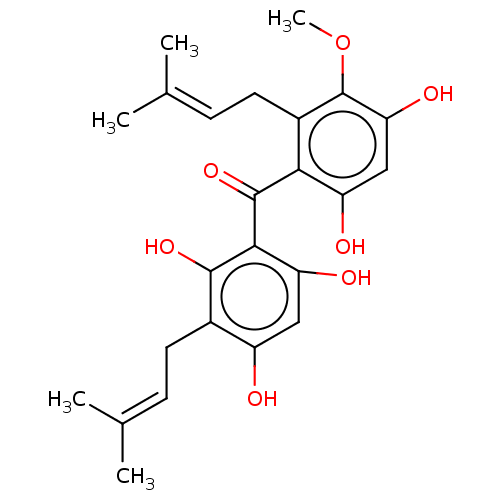
Affinity DataIC50: 2.59E+4nMAssay Description:Inhibitory activity against NSMase (neutral sphingomyelinase)More data for this Ligand-Target Pair
Affinity DataIC50: 2.77E+4nMAssay Description:Inhibitory activity against NSMase (neutral sphingomyelinase)More data for this Ligand-Target Pair
Affinity DataIC50: 1.14E+5nMAssay Description:Inhibitory activity against NSMase (neutral sphingomyelinase)More data for this Ligand-Target Pair
Affinity DataIC50: 1.14E+5nMAssay Description:Inhibitory activity against NSMase (neutral sphingomyelinase)More data for this Ligand-Target Pair
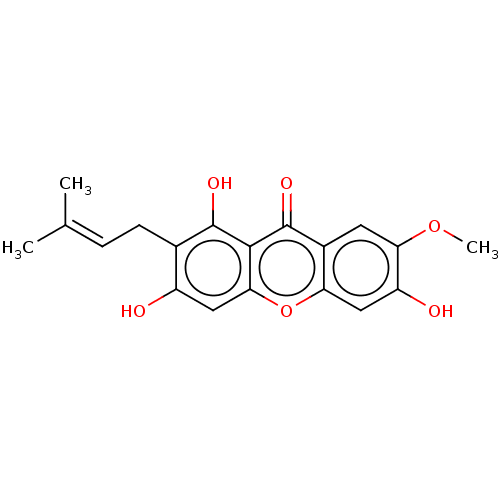
Affinity DataIC50: 1.81E+5nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.81E+5nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 2.02E+5nMAssay Description:Inhibitory activity against NSMase (neutral sphingomyelinase)More data for this Ligand-Target Pair
Affinity DataIC50: 2.92E+5nMAssay Description:Inhibitory activity against NSMase (neutral sphingomyelinase)More data for this Ligand-Target Pair
Affinity DataIC50: 3.77E+5nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 3.77E+5nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+5nMAssay Description:Inhibitory activity of the compound against neutral sphingomyelinase (N-SMase) from bovine brain microsomesMore data for this Ligand-Target Pair