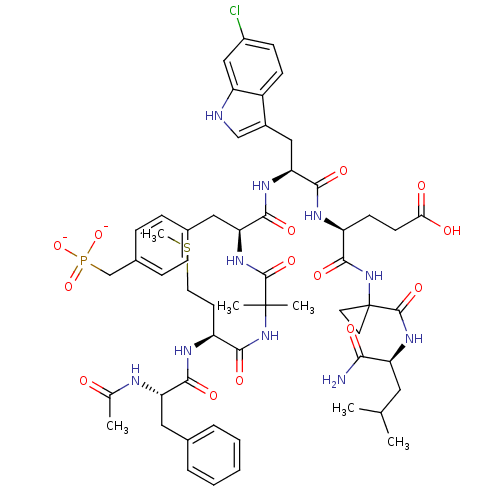
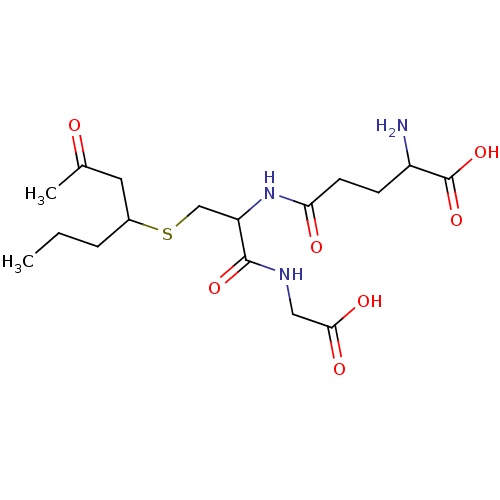
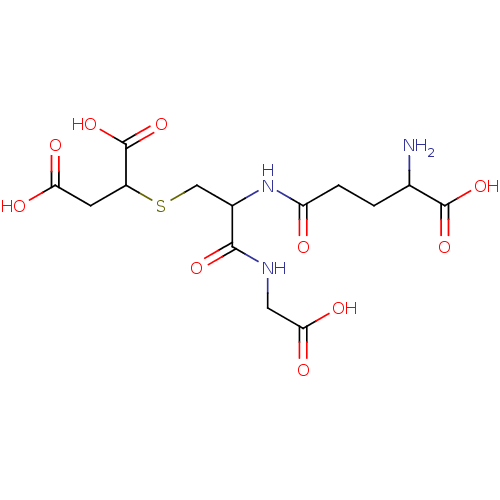
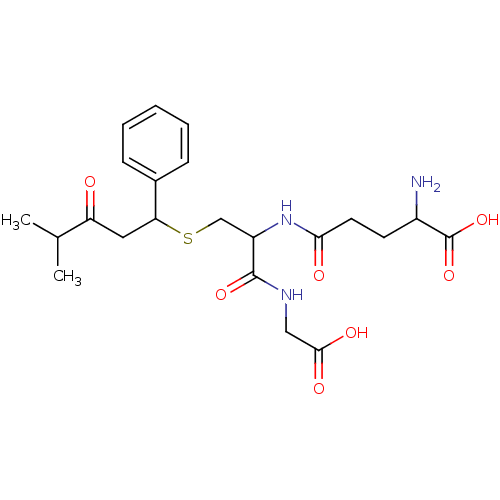
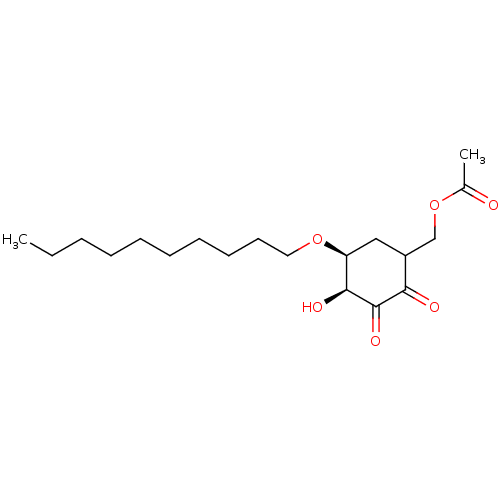
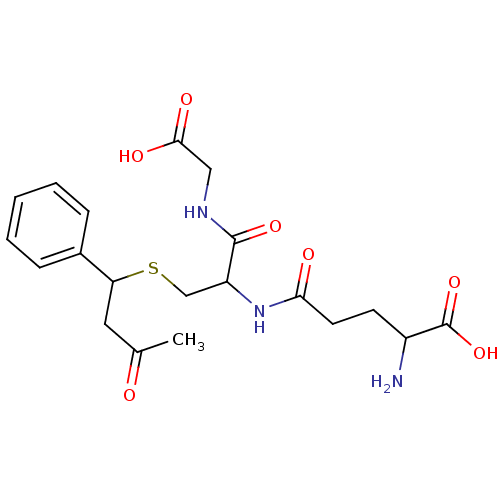
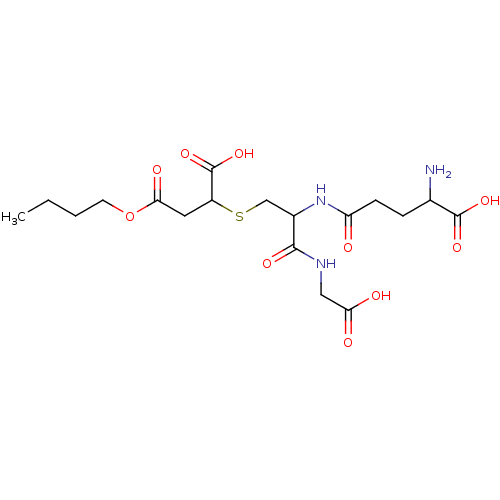
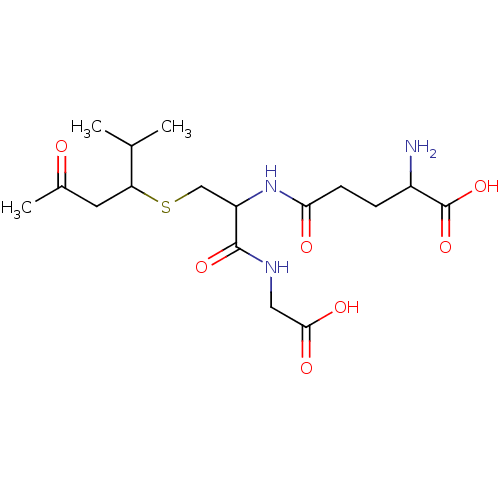
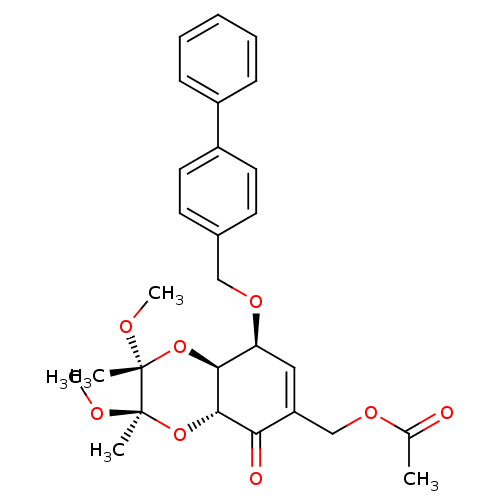
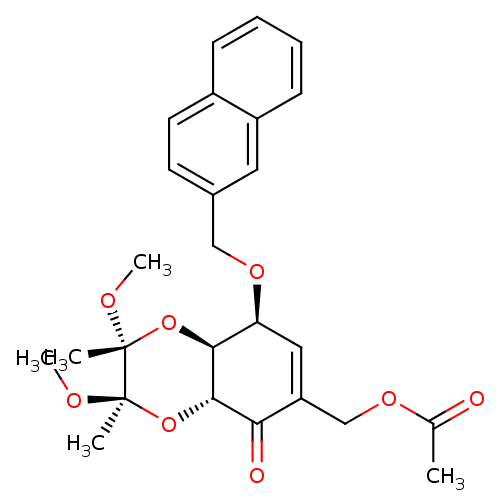
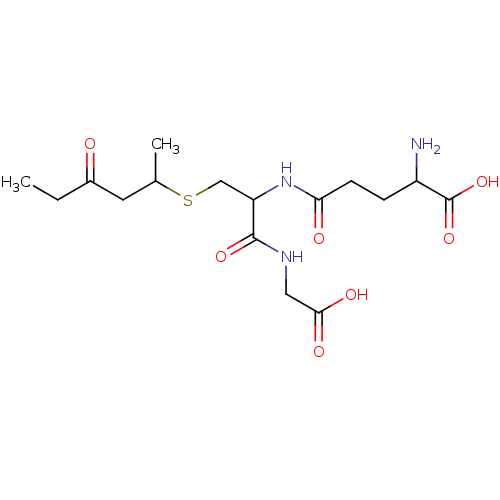
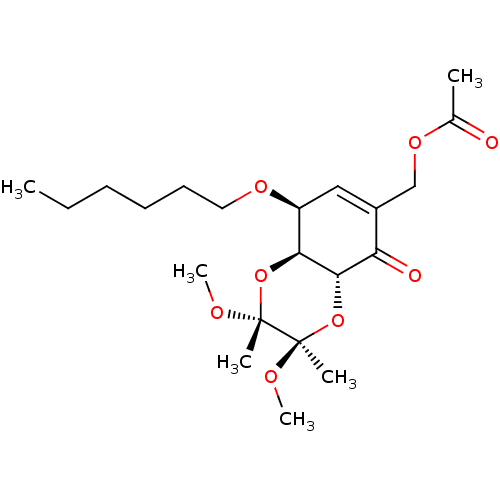
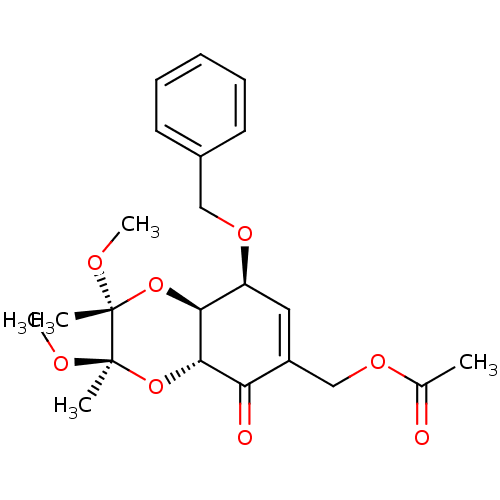
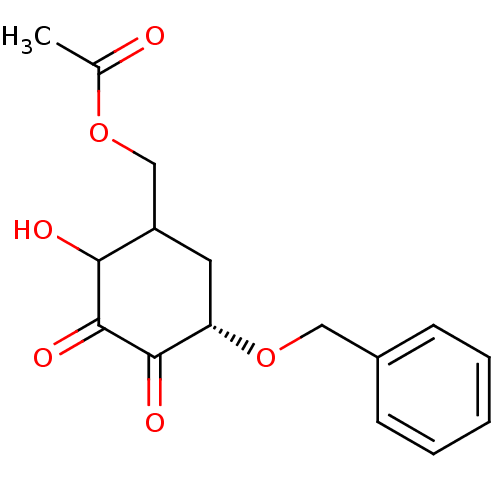
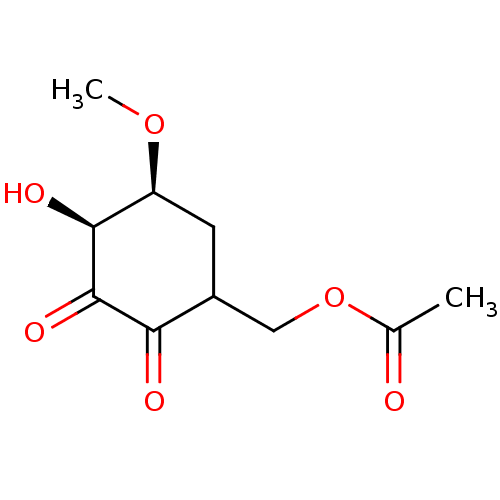
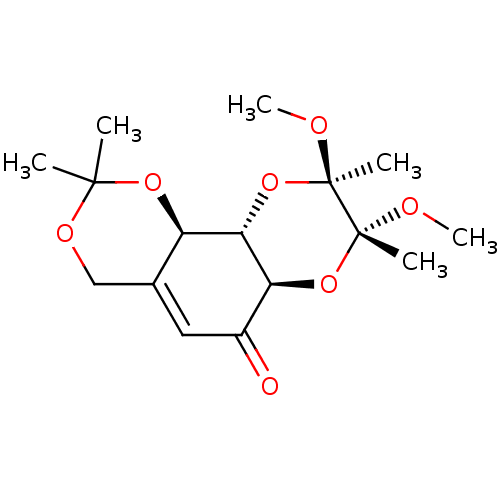
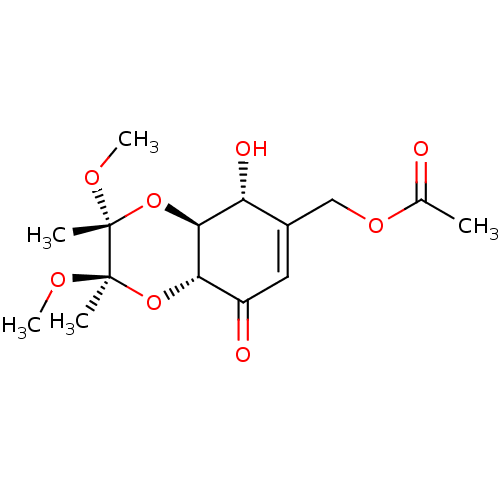
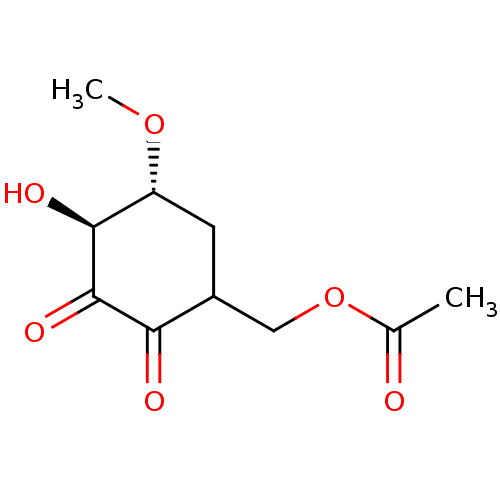
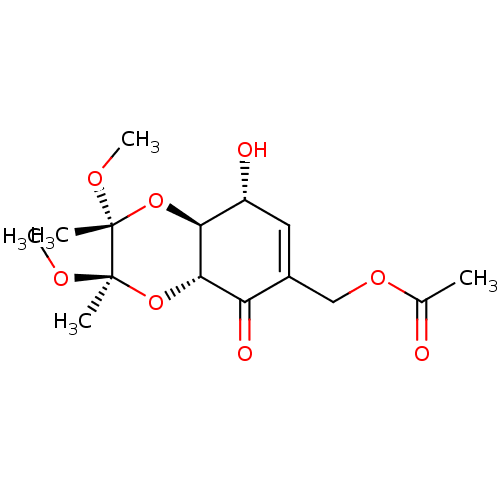
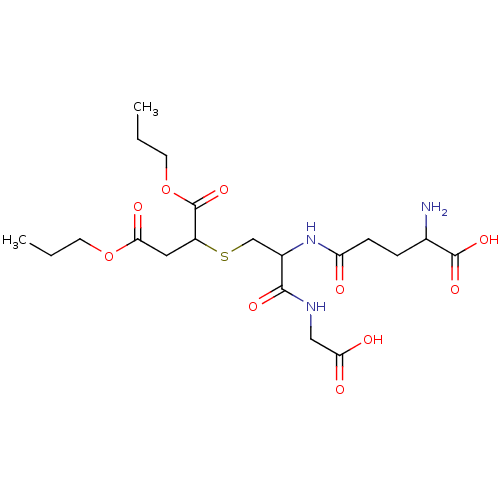
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of p53 binding to Glutathione S-transferase 2 (hdm2-GST)More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.10E+3nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 8.40E+3nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.91E+4nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.19E+4nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.31E+4nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.78E+4nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.10E+4nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.80E+4nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 6.60E+4nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 6.63E+4nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 7.76E+4nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 9.00E+4nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 9.30E+4nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 9.42E+4nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 9.63E+4nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.43E+5nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.79E+5nMAssay Description:Inhibition of human GSTA2 using GSH as substrate after 3 mins by spectrophotometryMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.20E+5nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair
TargetGlutathione S-transferase A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.50E+5nMAssay Description:The compound was tested for it's inhibitory activity against Onchocerca volvulus Glutathione S-transferase 2More data for this Ligand-Target Pair