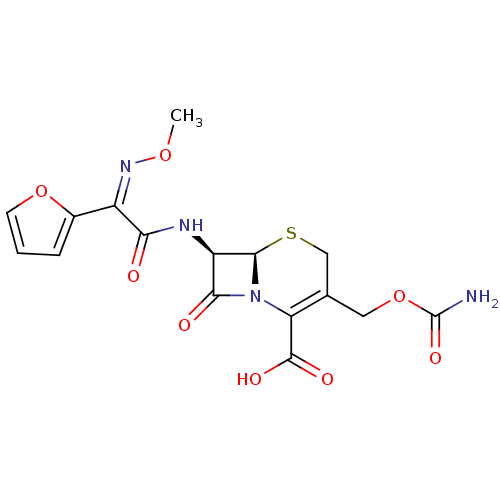
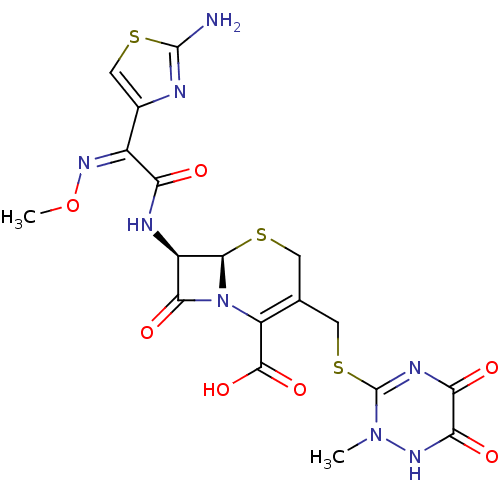
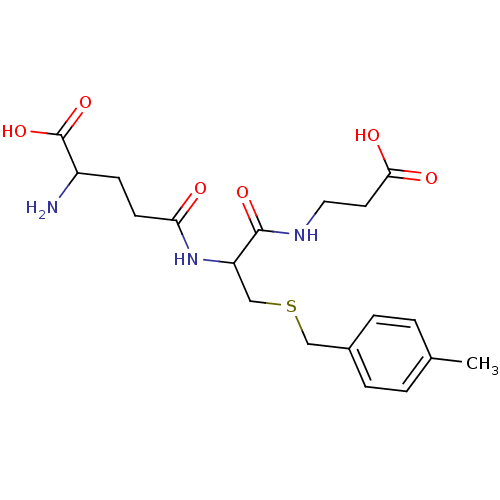
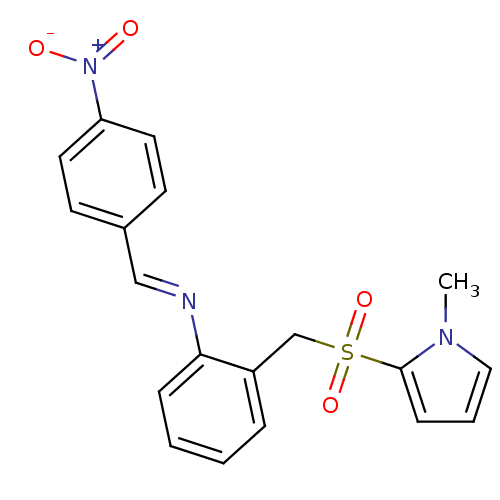
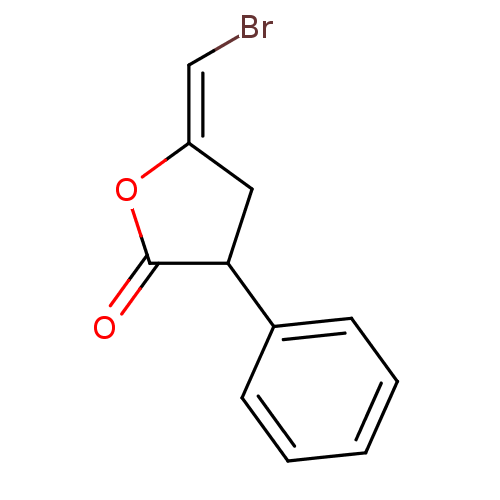
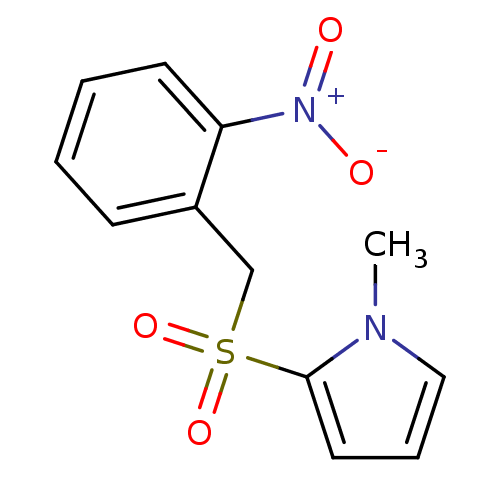
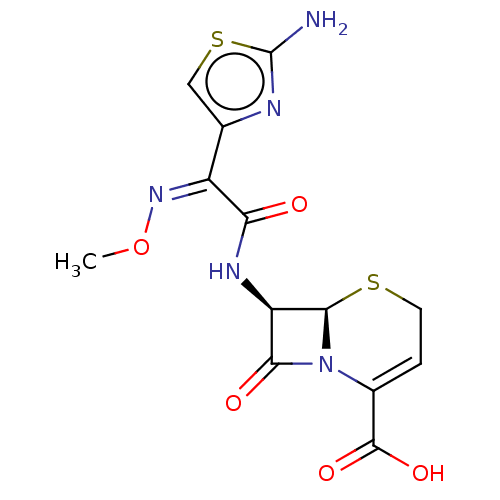
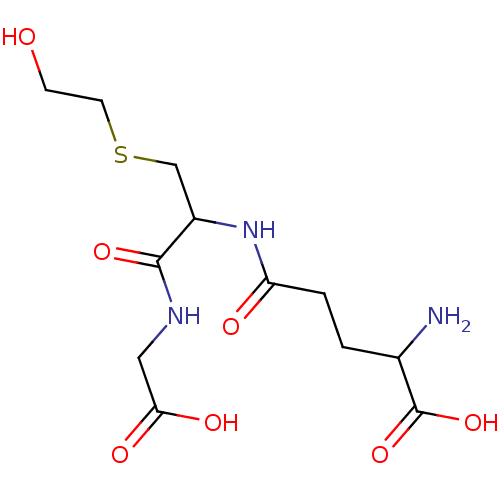
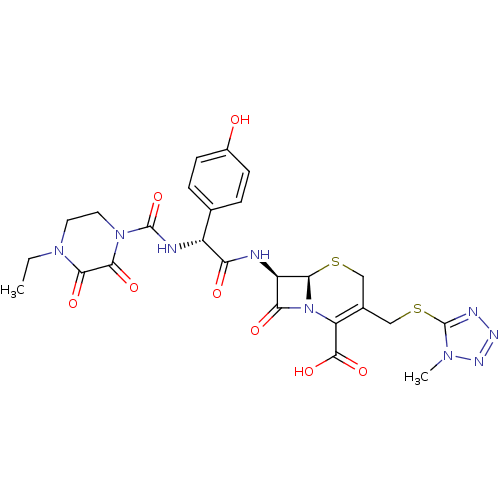
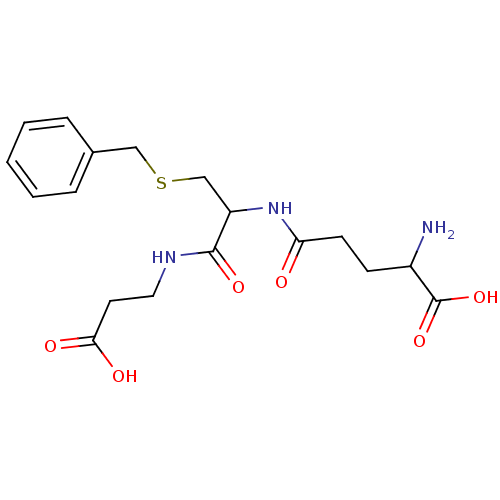
Affinity DataKi: 120nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase PMore data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Time dependent inhibition of GSTP1-1 (unknown origin) assessed as inhibition constant using reduced GSH and CDNB as substrate by spectrophotometric m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 360nMAssay Description:Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graphMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 380nMAssay Description:Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graphMore data for this Ligand-Target Pair
Affinity DataKi: 420nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase PMore data for this Ligand-Target Pair
Affinity DataKi: 490nMAssay Description:Inhibition constant against glutathione S-transferase pi using GSH (0.1-3mM), 1 mM 1-chloro-2,4-dinitrobenzeneMore data for this Ligand-Target Pair
Affinity DataKi: 660nMAssay Description:Inhibition constant against glutathione S-transferase pi using GSH (0.1-3mM), 1 mM 1-chloro-2,4-dinitrobenzeneMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 840nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 850nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase PMore data for this Ligand-Target Pair
Affinity DataKi: 1.11E+3nMAssay Description:Inhibition constant against glutathione S-transferase pi using GSH (0.1-3mM), 1 mM 1-chloro-2,4-dinitrobenzeneMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 1.47E+3nMAssay Description:Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graphMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by 1-chloro-2,4-dinitrobenzene competitive assayMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by 1-chloro-2,4-dinitrobenzene competitive assayMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by 1-chloro-2,4-dinitrobenzene competitive assayMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 1.75E+3nMAssay Description:Competitive inhibition of human GSTA1 activity by double reciprocal Lineweaver-Burk graphMore data for this Ligand-Target Pair
Affinity DataKi: 1.85E+3nMAssay Description:Time dependent inhibition of GSTP1-1 (unknown origin) assessed as inhibition constant using reduced GSH and CDNB as substrate by spectrophotometric m...More data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 5.80E+3nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 6.10E+3nMAssay Description:Inhibitory activity of the compound for human Glutathione S-transferase P was determinedMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 8.10E+3nMAssay Description:Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by glutathione competitive assayMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 8.10E+3nMAssay Description:Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by glutathione competitive assayMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 8.10E+3nMAssay Description:Inhibition of human GSTA1-1 expressed in Escherichia coli BL21 (DE3) by glutathione competitive assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase PMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 1.47E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzymeMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 2.43E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 3.24E+4nM IC50: 5.64E+4nMpH: 6.5Assay Description:Enzymatic activity was determined spectrophotometrically by measuring the conjugation of CDNB with GSH. The 1 ml assay mixture contained 0.5 mM CDNB,...More data for this Ligand-Target Pair
Affinity DataKi: 3.54E+4nM IC50: 6.18E+4nMpH: 6.5Assay Description:Enzymatic activity was determined spectrophotometrically by measuring the conjugation of CDNB with GSH. The 1 ml assay mixture contained 0.5 mM CDNB,...More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase PMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 4.30E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzymeMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 4.30E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzymeMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 7.10E+4nMAssay Description:Competitive inhibition of recombinant human GSTA1-1 expressed in Escherichia coli BL21 (DE3) using CDNB as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.24E+5nMAssay Description:Inhibitory activity of the compound for human glutathione S-transferase-pi isozyme was determinedMore data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 1.35E+5nMAssay Description:Competitive inhibition of recombinant human GSTA1-1 expressed in Escherichia coli BL21 (DE3) using CDNB as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.65E+5nM IC50: 2.78E+5nMpH: 6.5Assay Description:Enzymatic activity was determined spectrophotometrically by measuring the conjugation of CDNB with GSH. The 1 ml assay mixture contained 0.5 mM CDNB,...More data for this Ligand-Target Pair
Affinity DataKi: 1.87E+5nMAssay Description:Inhibition constant against glutathione S-transferase pi using GSH (0.1-3mM), 1 mM 1-chloro-2,4-dinitrobenzeneMore data for this Ligand-Target Pair
Affinity DataKi: 2.67E+5nM IC50: 4.84E+5nMpH: 6.5Assay Description:Enzymatic activity was determined spectrophotometrically by measuring the conjugation of CDNB with GSH. The 1 ml assay mixture contained 0.5 mM CDNB,...More data for this Ligand-Target Pair
TargetGlutathione S-transferase A1(Homo sapiens (Human))
Agricultural University of Athens
Curated by ChEMBL
Agricultural University of Athens
Curated by ChEMBL
Affinity DataKi: 3.60E+5nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 5.50E+5nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase PMore data for this Ligand-Target Pair
Affinity DataKi: 7.10E+5nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase PMore data for this Ligand-Target Pair

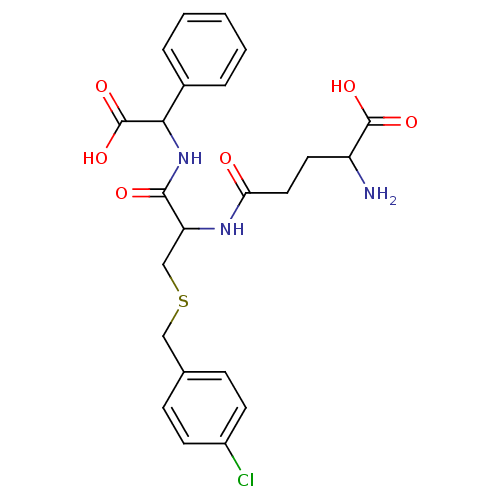


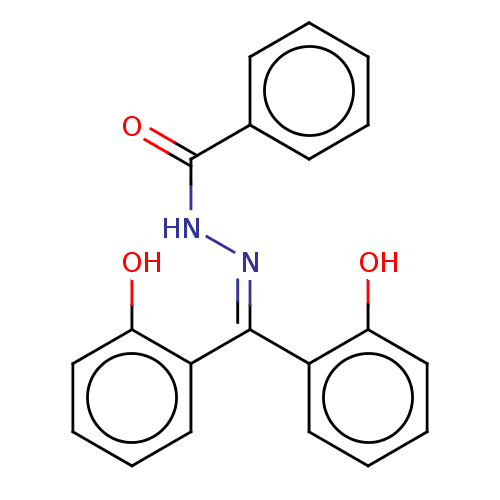
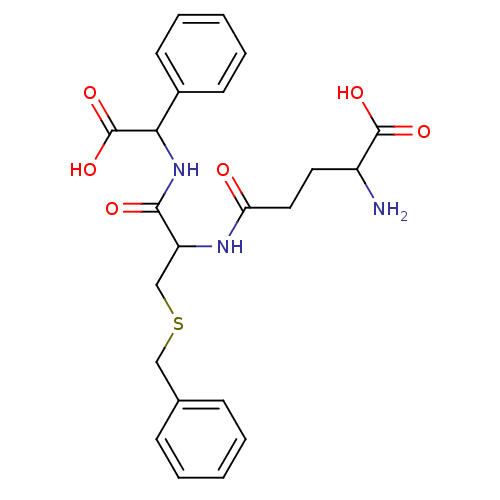

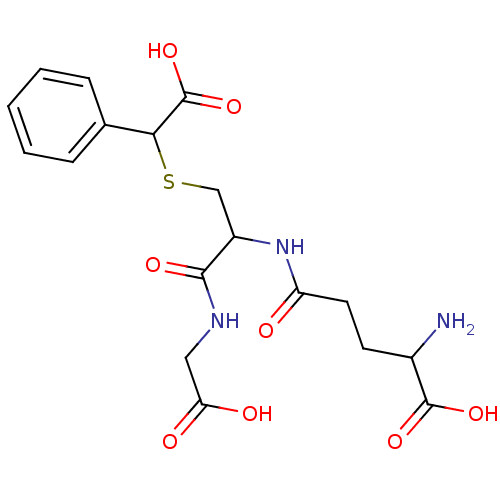

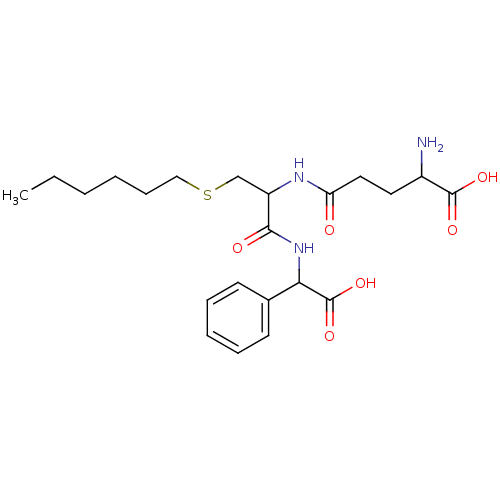
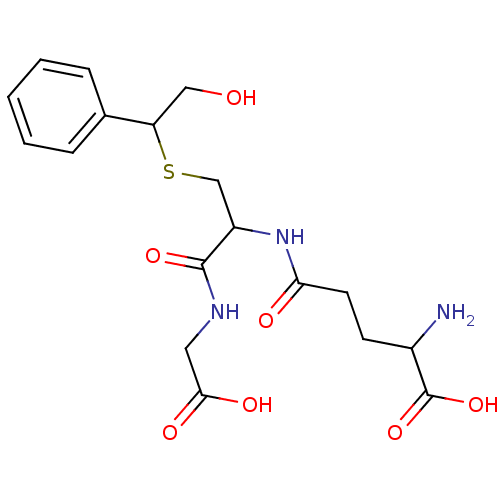

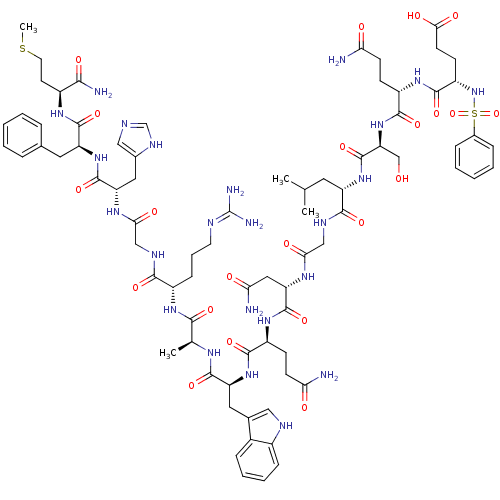

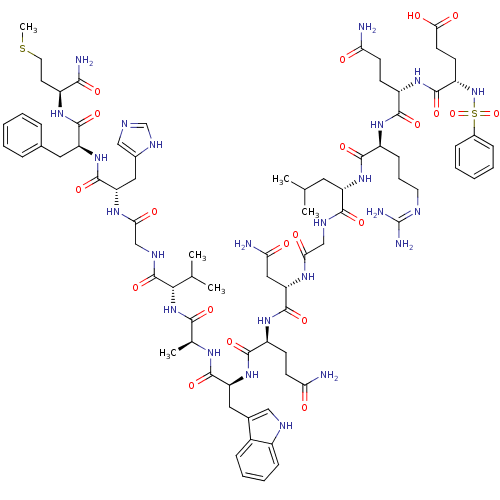
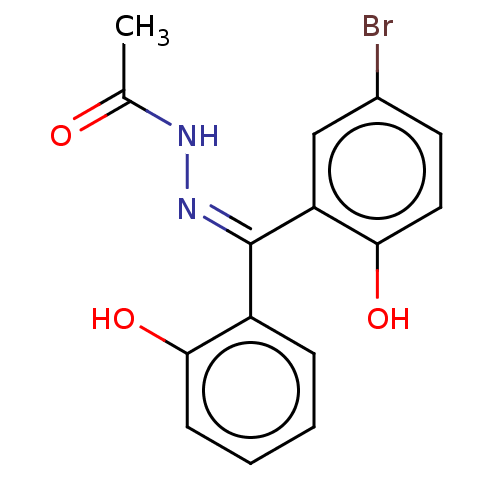
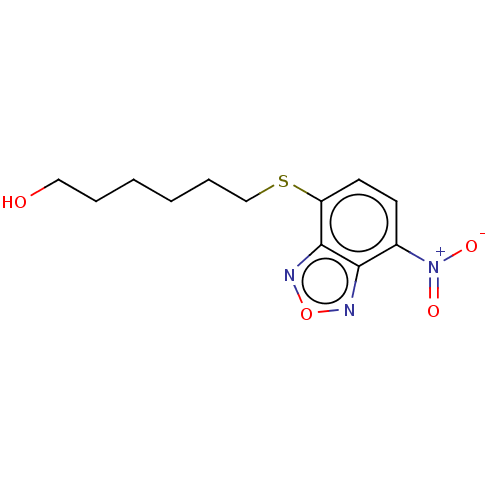
 3D Structure (crystal)
3D Structure (crystal)