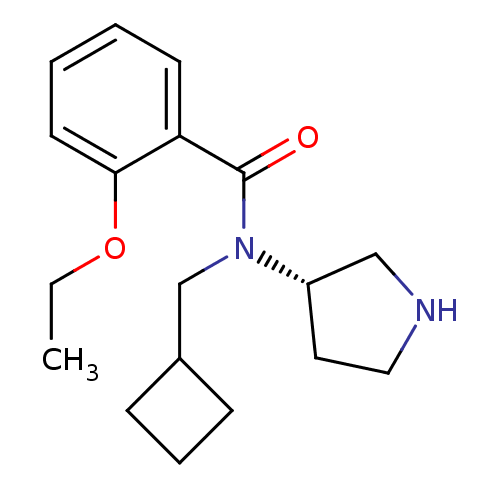
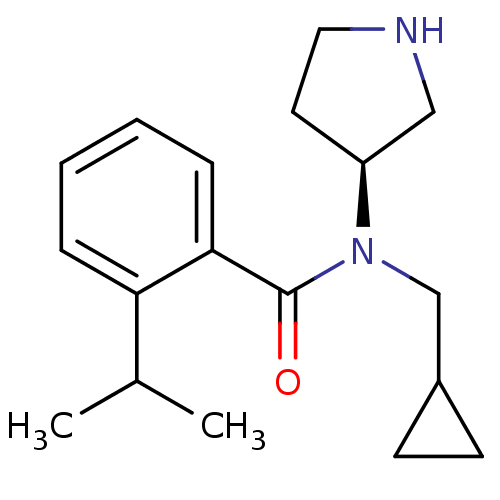
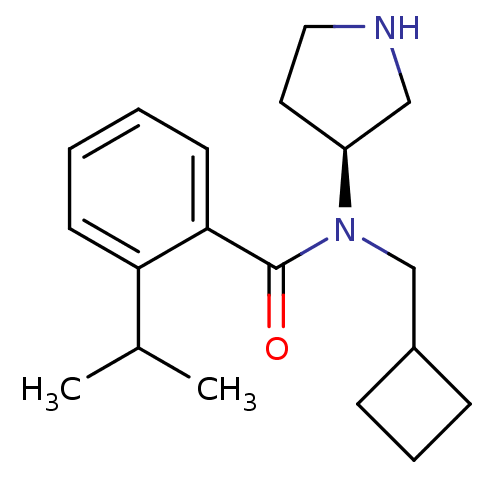
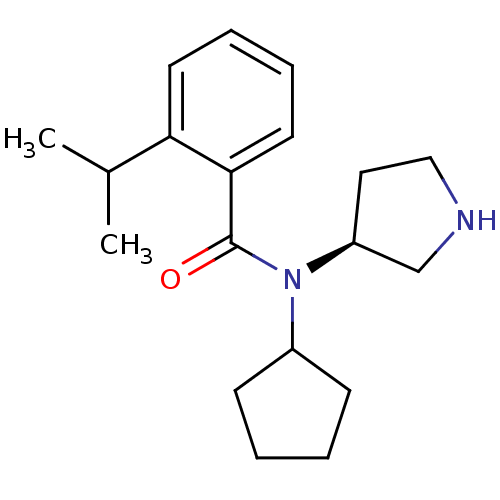
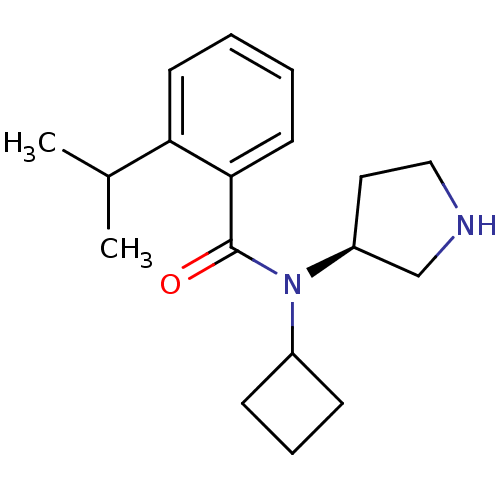
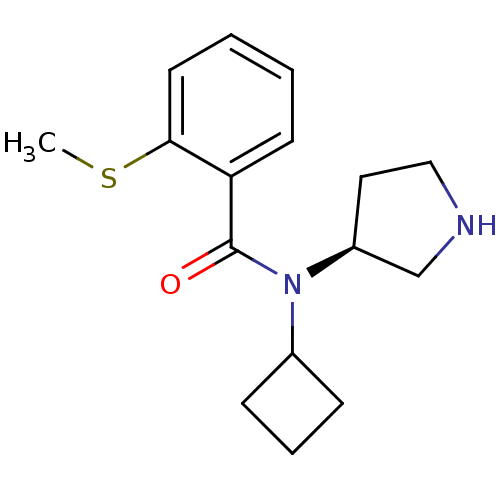
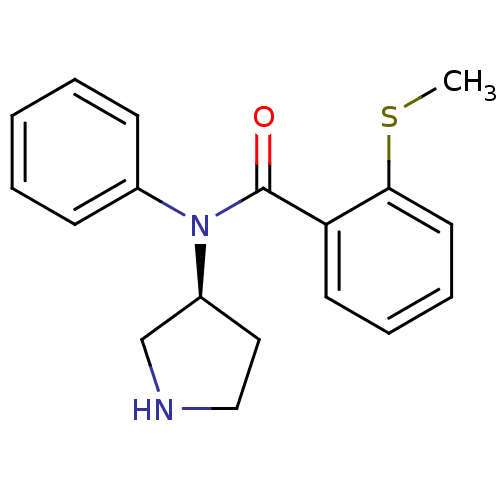
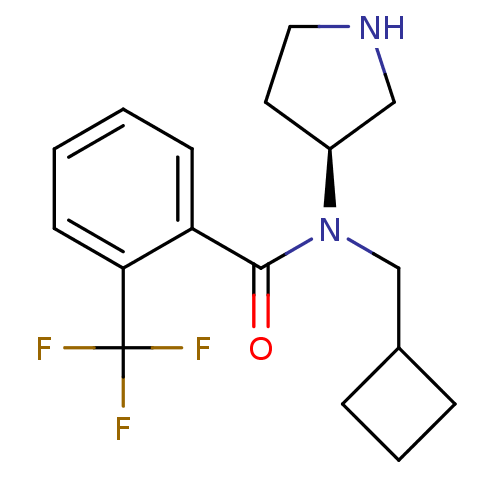
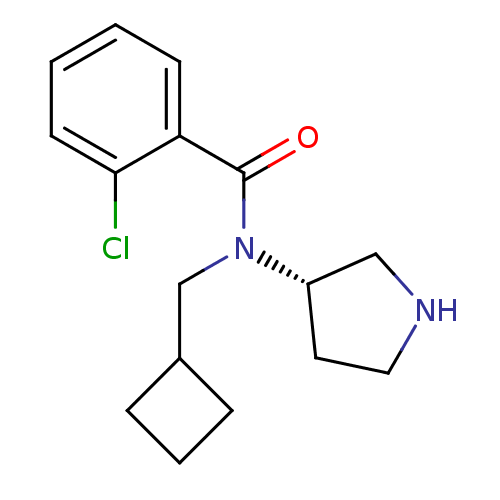
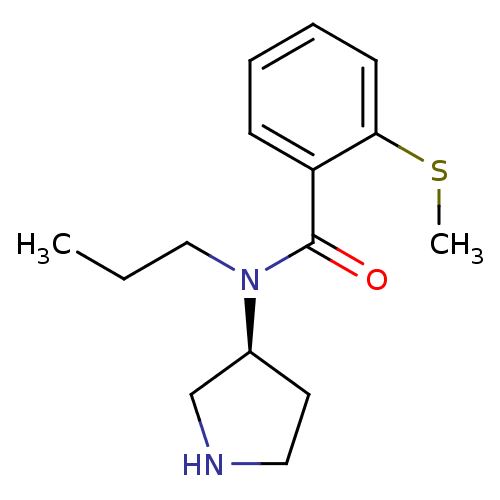
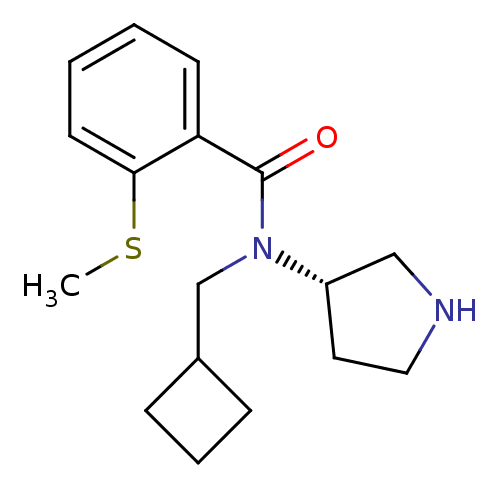
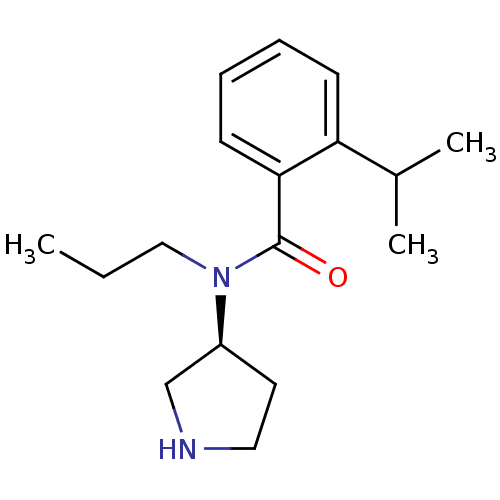
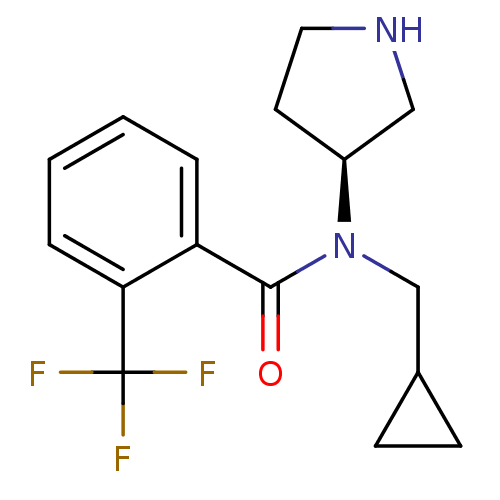
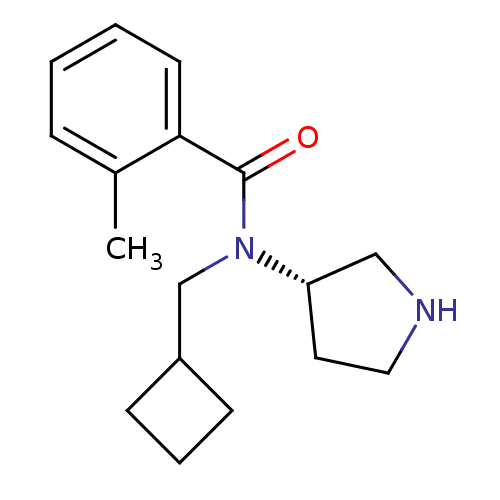
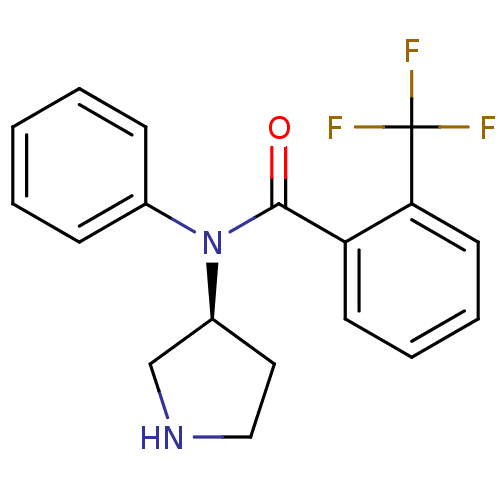
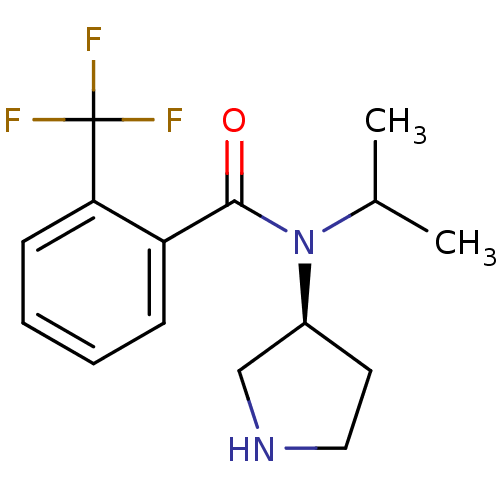
Found 176 hits of Enzyme Inhibition Constant Data
Found 176 hits of Enzyme Inhibition Constant Data
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.68E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.76E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.82E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.85E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.87E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.90E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]dofetilide from human ERGMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of CYP3A4 using felodipine as substrateMore data for this Ligand-Target Pair
TargetSodium channel protein type 5 subunit alpha(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.60E+4nMAssay Description:Blockade of Nav1.5 sodium channelMore data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.12E+4nMAssay Description:Inhibition of CYP3A4 using testosterone as substrateMore data for this Ligand-Target Pair