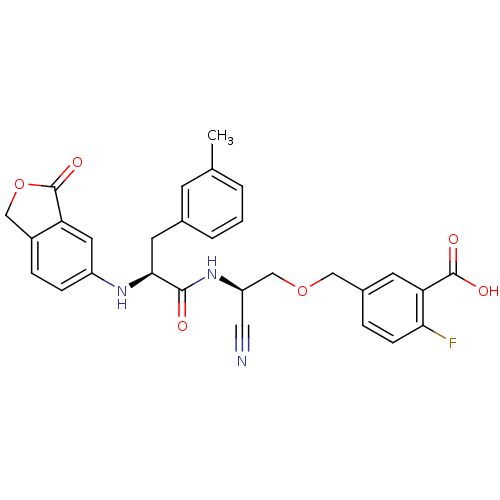
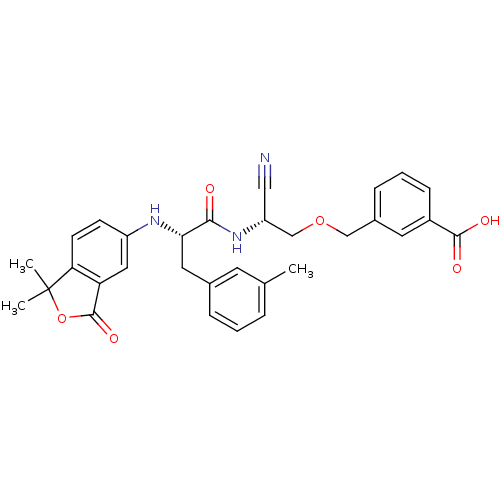
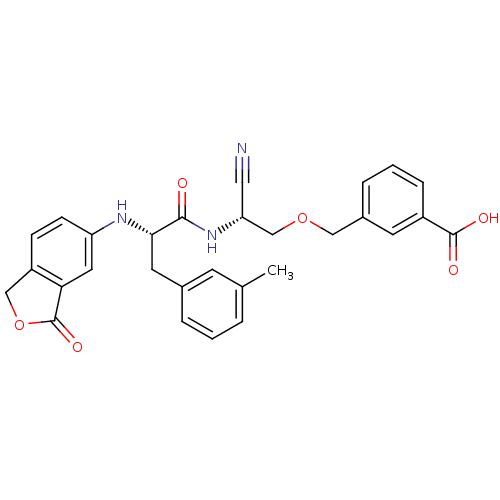
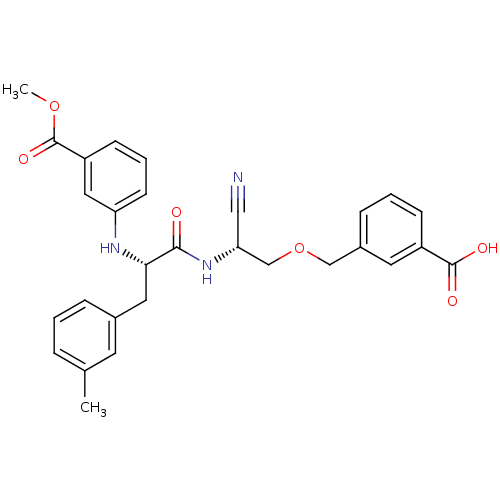
Found 41 hits of Enzyme Inhibition Constant Data
Found 41 hits of Enzyme Inhibition Constant Data
Affinity DataIC50: 4.10nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 6.5nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 8.60nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 8.70nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 9.30nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 49nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 194nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
Affinity DataIC50: 282nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 560nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
Affinity DataIC50: 710nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
Affinity DataIC50: 780nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
Affinity DataIC50: 832nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibtitory activity against cathepsin B (catB)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibtitory activity against cathepsin S (catS)More data for this Ligand-Target Pair
TargetProcathepsin L(Homo sapiens (Human))
Novartis Institute of Biomedical Research
Curated by ChEMBL
Novartis Institute of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibtitory activity against cathepsin L (catL)More data for this Ligand-Target Pair