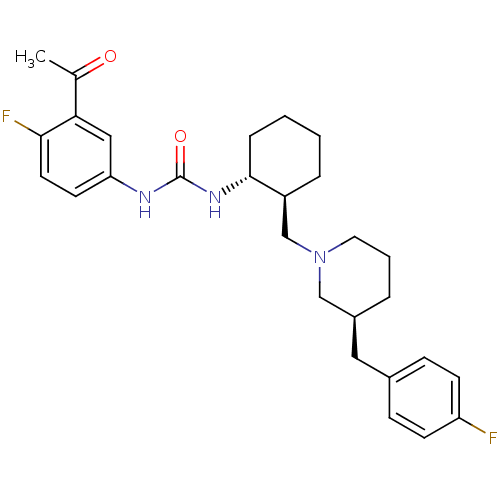
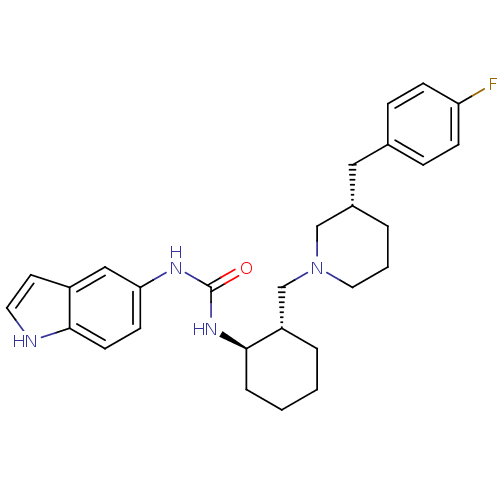
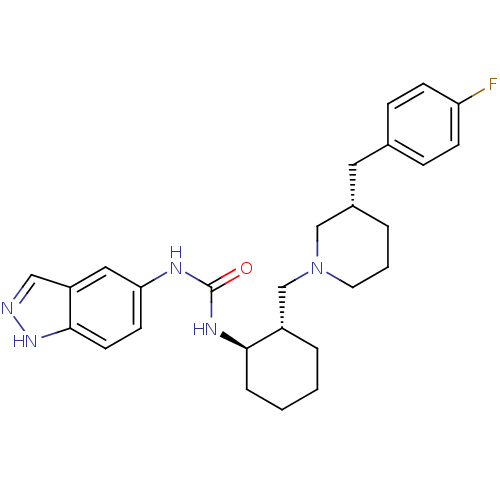
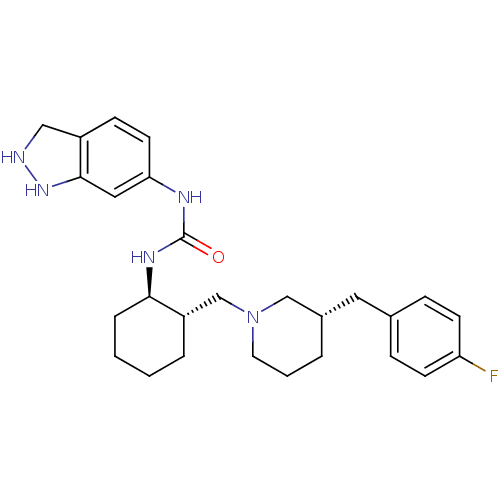
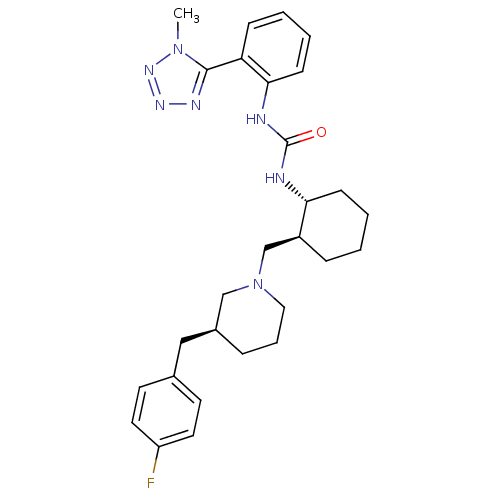
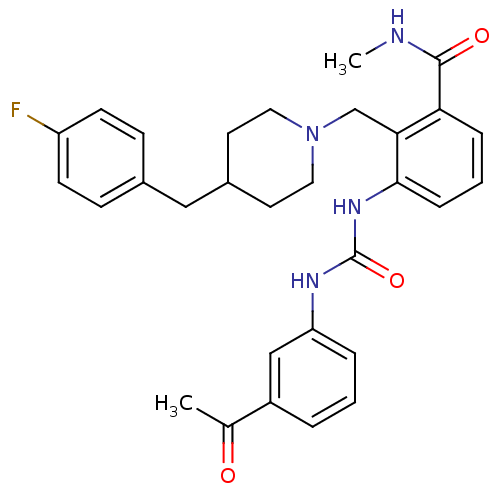
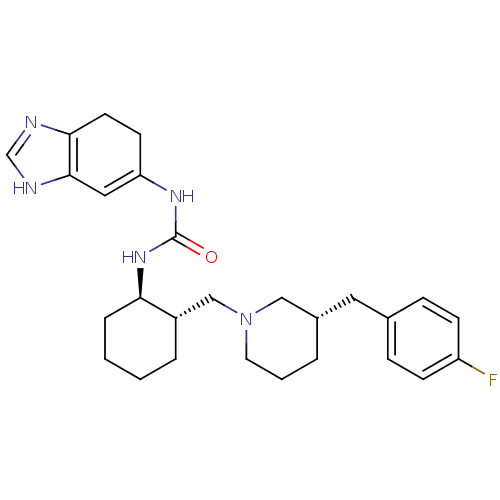
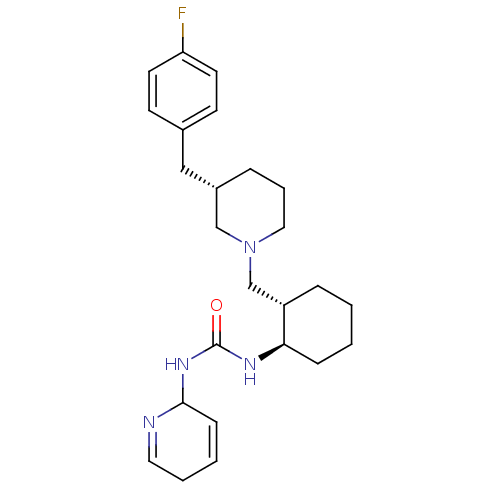
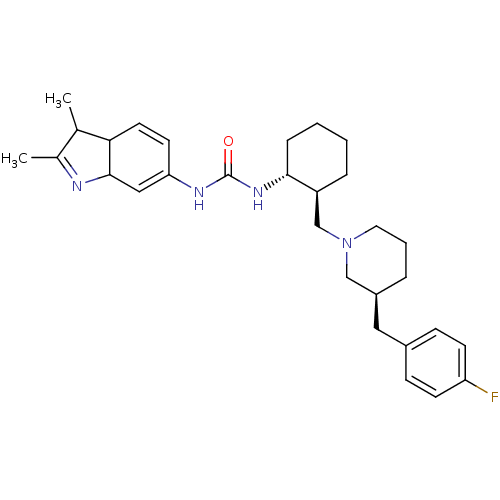
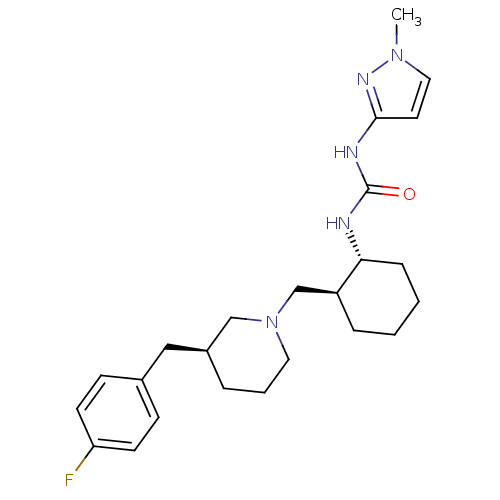
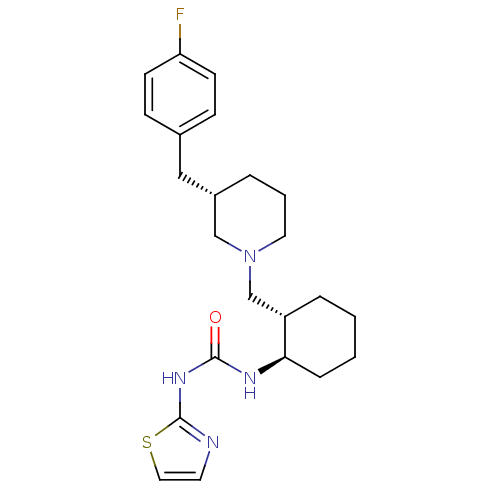
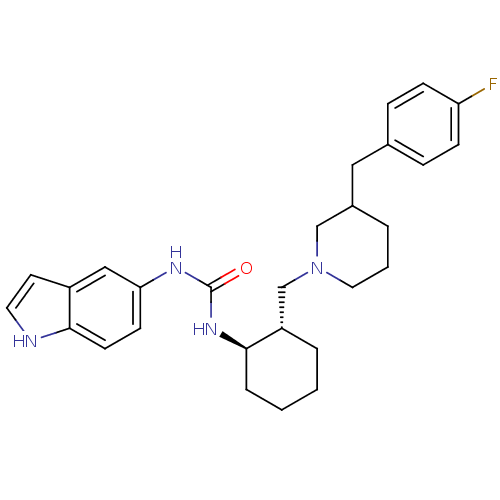
Found 125 hits of Enzyme Inhibition Constant Data
Found 125 hits of Enzyme Inhibition Constant Data
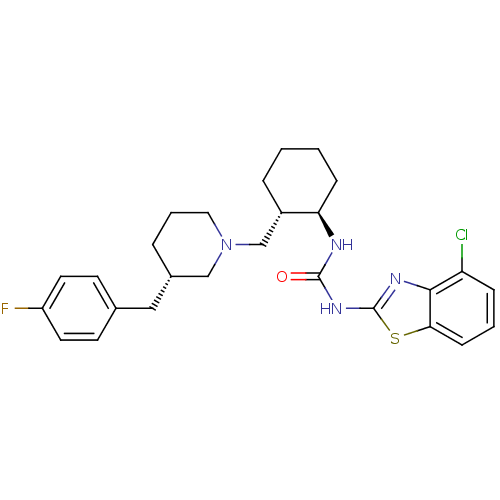
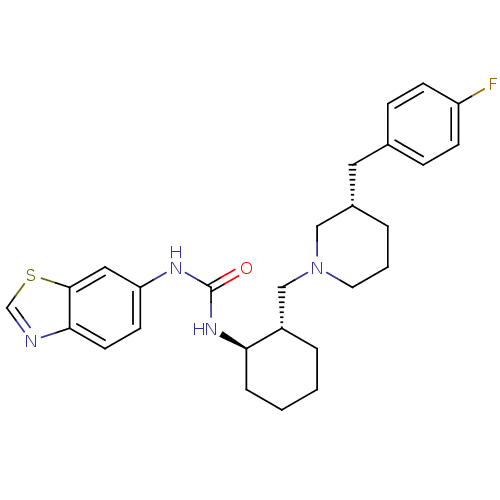
TargetC-C chemokine receptor type 3(Rattus norvegicus)
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.00700nMAssay Description:Inhibition of eotaxin-induced chemotaxis of rat eosinophilsMore data for this Ligand-Target Pair
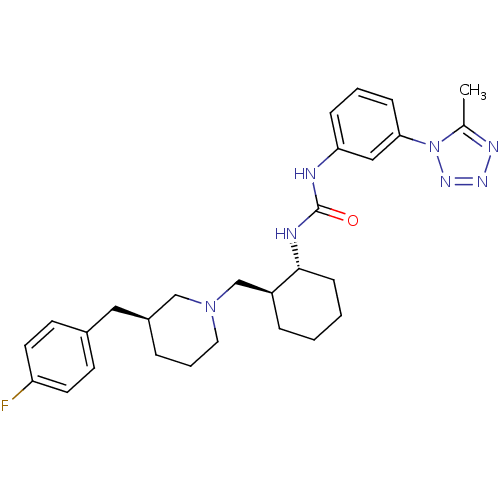
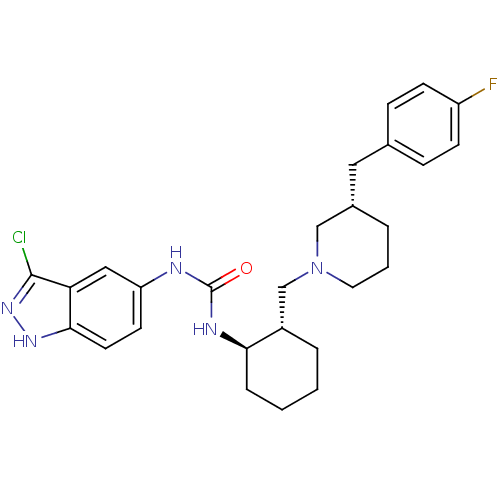
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.00700nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
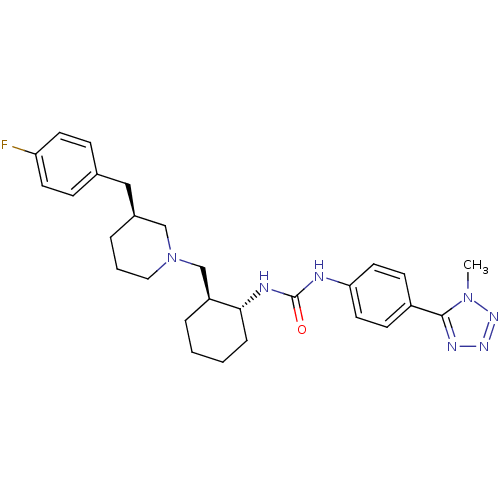
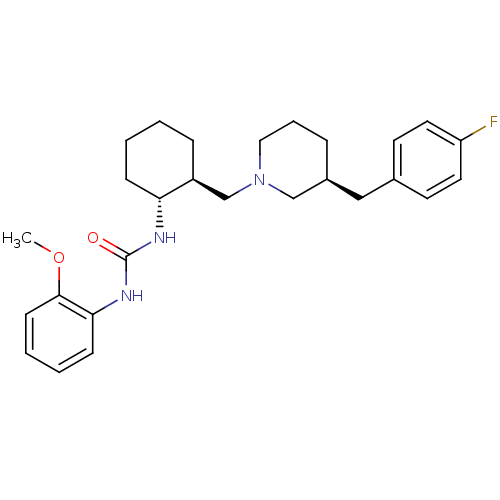
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0100nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
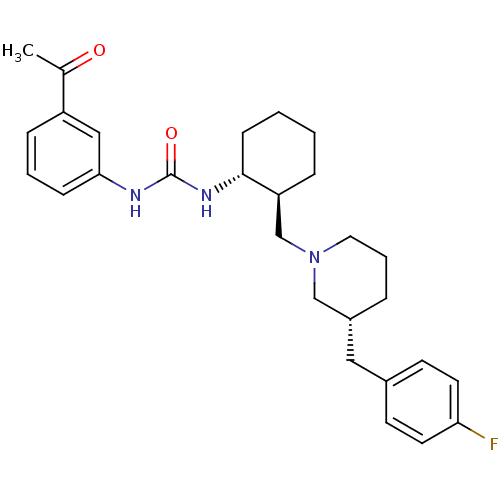
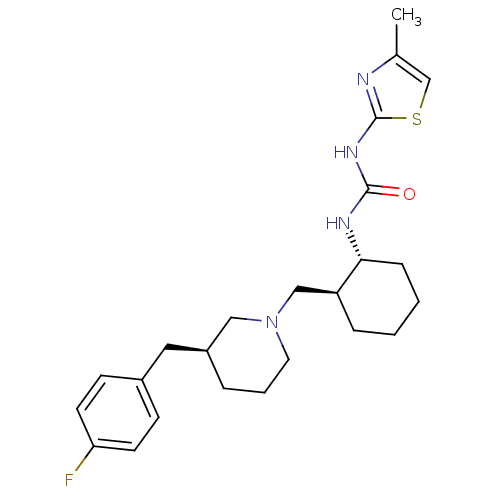
TargetC-C chemokine receptor type 3(Rattus norvegicus)
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0100nMAssay Description:Inhibition of eotaxin-induced chemotaxis of rat eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0150nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0160nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0340nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0390nMAssay Description:Inhibition of eotaxin-induced chemotaxis of Cynomolgus monkey eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0420nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0450nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0600nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.0900nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.25nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.364nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of [125I]-eotaxin binding to human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of [125I]-eotaxin binding to human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of [125I]-eotaxin binding to human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of calcium mobilization in human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of [125I]eotaxin binding to human C-C chemokine receptor type 3 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of eotaxin-induced chemotaxis of human eosinophilsMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 3(Homo sapiens (Human))
Pharmaceutical Research Institute
Curated by ChEMBL
Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of calcium mobilization in human eosinophilsMore data for this Ligand-Target Pair