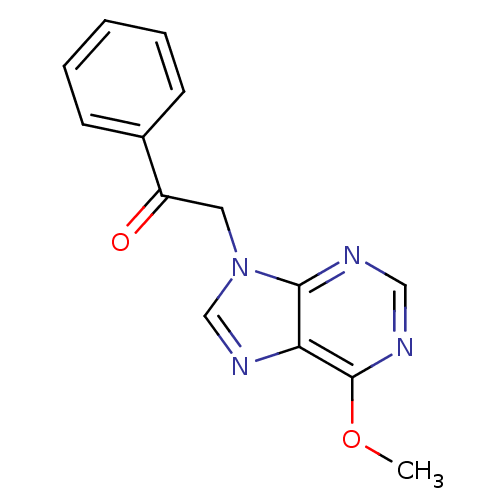
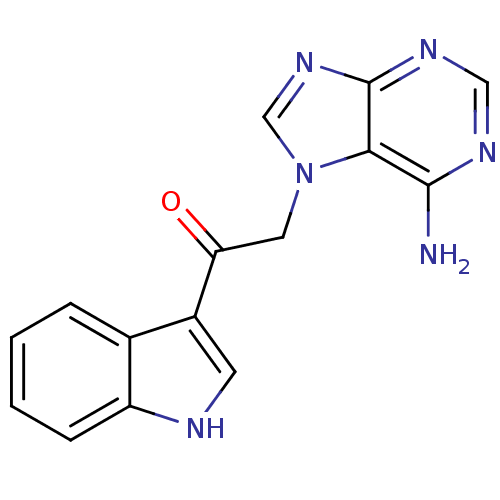
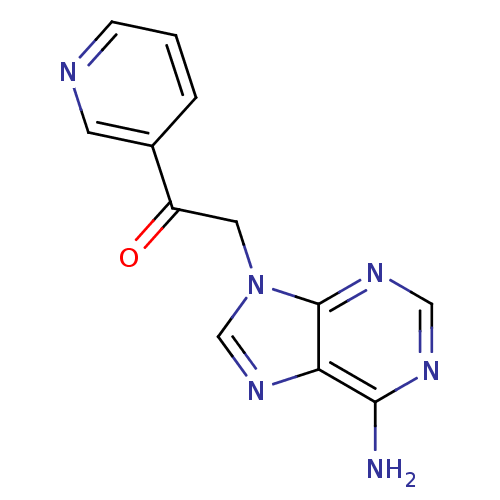
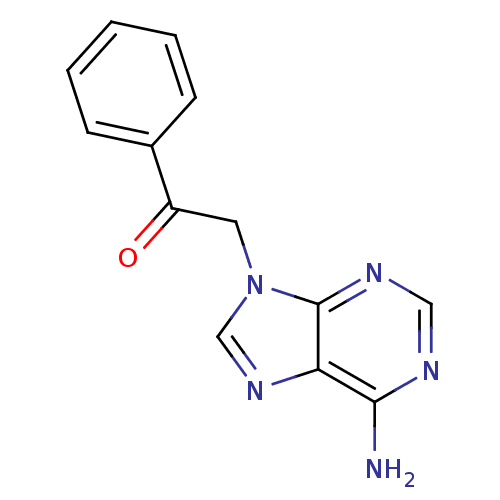
Found 16 hits of Enzyme Inhibition Constant Data
Found 16 hits of Enzyme Inhibition Constant Data
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of VEGFR2More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of VEGFR2More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 3.50E+4nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 3(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 4.50E+4nMAssay Description:Inhibition of VEGFR3More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 5.80E+4nMAssay Description:Inhibition of VEGFR2More data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+4nMAssay Description:Inhibition of Aurora BMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 7.50E+4nMAssay Description:Inhibition of SRCMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 7.50E+4nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 8.10E+4nMAssay Description:Inhibition of VEGFR2More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 8.20E+4nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 8.20E+4nMAssay Description:Inhibition of SRCMore data for this Ligand-Target Pair
Affinity DataIC50: 8.50E+4nMAssay Description:Inhibition of TIE2More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 8.90E+4nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 9.30E+4nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Eberhard-Karls-University
Curated by ChEMBL
Eberhard-Karls-University
Curated by ChEMBL
Affinity DataIC50: 9.50E+4nMAssay Description:Inhibition of ERBB2More data for this Ligand-Target Pair
Affinity DataIC50: 9.50E+4nMAssay Description:Inhibition of TIE2More data for this Ligand-Target Pair