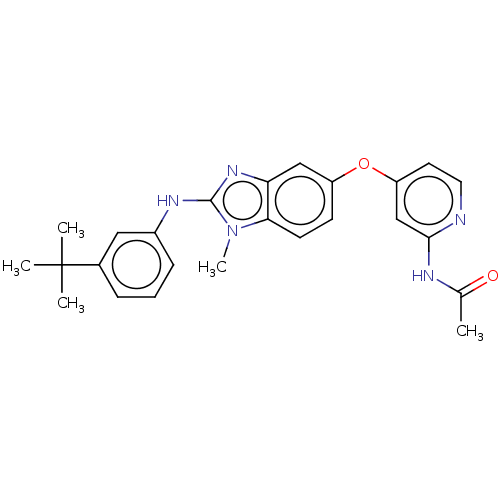
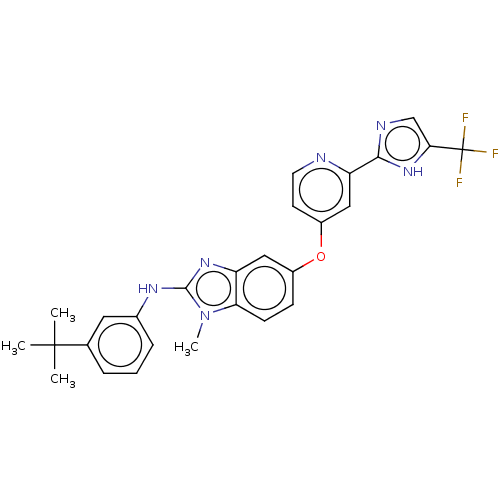
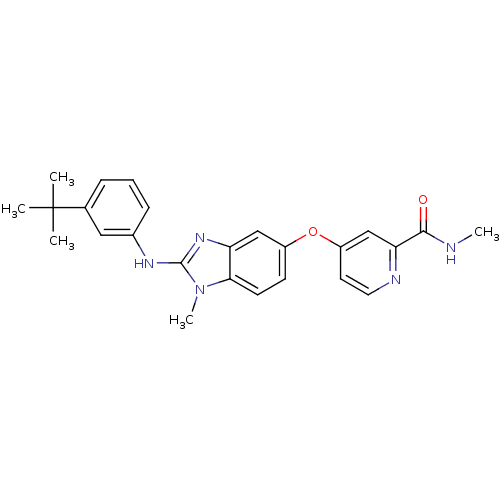
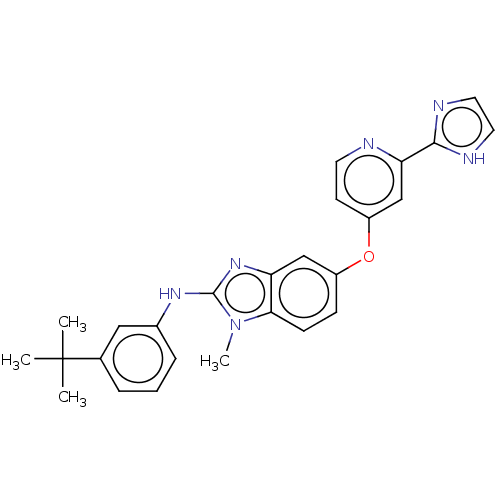
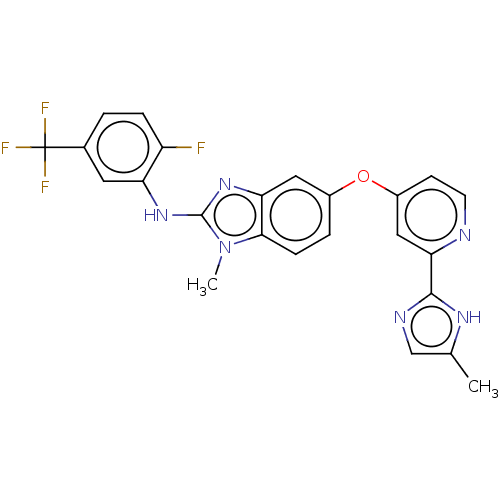
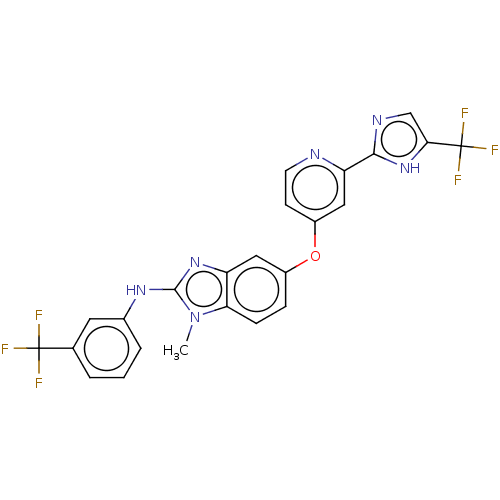
Found 58 hits of Enzyme Inhibition Constant Data
Found 58 hits of Enzyme Inhibition Constant Data
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of Histamine H1 receptorMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of Histamine H1 receptorMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fyn(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferaseMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferaseMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1/2/3(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Binding affinity towards Kappa opioid receptorMore data for this Ligand-Target Pair
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Reduced farnesylation of H-ras transformed NIH3T3 cellsMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fyn(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferaseMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1/2/3(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Binding affinity towards Kappa opioid receptorMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of Histamine H1 receptorMore data for this Ligand-Target Pair
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Reduced farnesylation of H-ras transformed NIH3T3 cellsMore data for this Ligand-Target Pair
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of kappa opioid receptorMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1/2/3(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition against rat hydroxytryptamine 3 receptorMore data for this Ligand-Target Pair
TargetMast/stem cell growth factor receptor Kit(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of histamine H1 receptorMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1/2/3(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fyn(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptideMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferaseMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferaseMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferaseMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 44nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Reduced farnesylation of H-ras transformed NIH3T3 cellsMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Binding affinity towards Kappa opioid receptorMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptideMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1/2/3(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptideMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 120nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Binding affinity towards Kappa opioid receptorMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fyn(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferaseMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 310nMAssay Description:Inhibition of Histamine H1 receptorMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 480nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 620nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 780nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNAMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptideMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibitory activity towards acetylcholine esterase (AChE)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptideMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:inhibitory concentration needed to to reduce the bovine GGTase-catalyzed incorporation of [3H]-FPP into a biotin-linked K-ras (B) decapeptideMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)