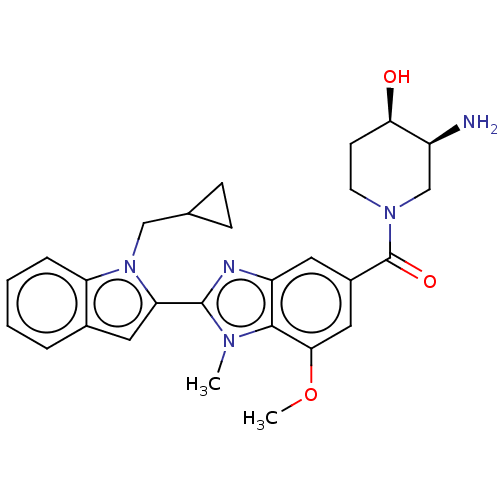
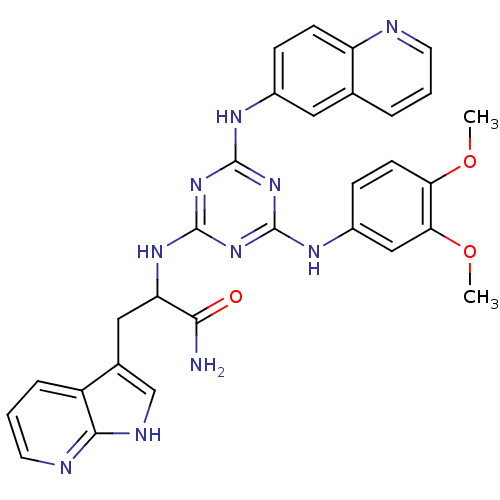
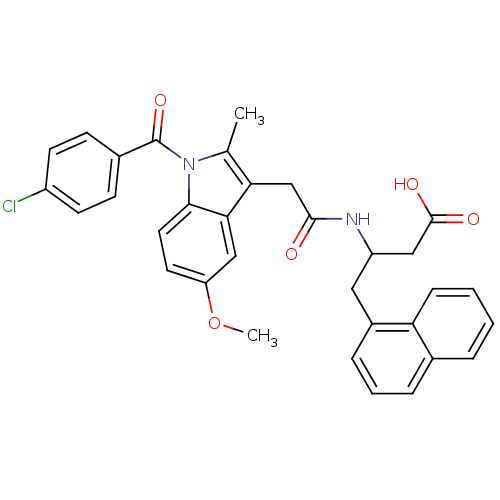
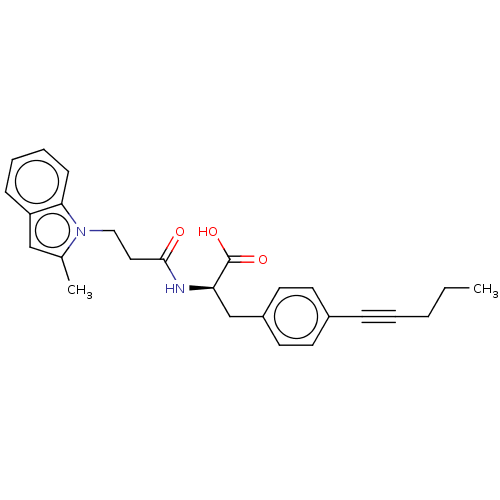
Found 24 hits of Enzyme Inhibition Constant Data
Found 24 hits of Enzyme Inhibition Constant Data
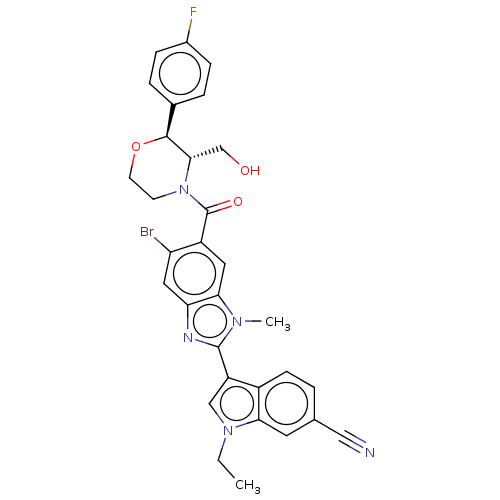
Affinity DataIC50: 0.0280nMAssay Description:Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0280nMAssay Description:Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 3(Homo sapiens (Human))
University of Utah
Curated by ChEMBL
University of Utah
Curated by ChEMBL
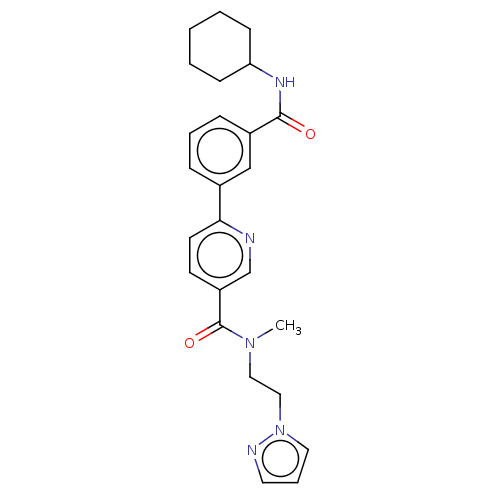
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant RIP3 (2 to 328 residues) expressed in baculovirus by ADP-glo assayMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 3(Homo sapiens (Human))
University of Utah
Curated by ChEMBL
University of Utah
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant RIP3 (2 to 328 residues) expressed in baculovirus by ADP-glo assayMore data for this Ligand-Target Pair
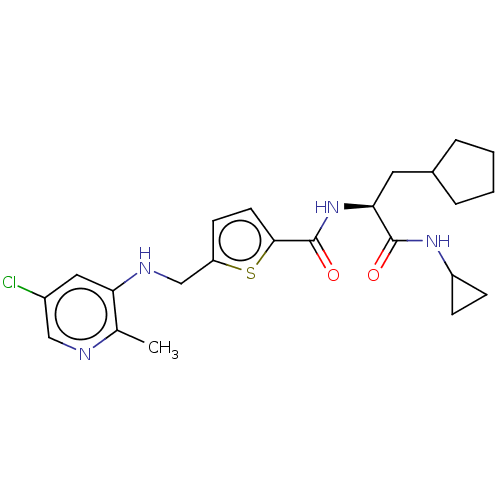
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human PDE12 (17 to 609 residues) expresssed in Escherichia coli BL21(DE3) cells using 2-5A as substrate assessed as AMP monomers and AT...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human PDE12 (17 to 609 residues) expresssed in Escherichia coli BL21(DE3) cells using 2-5A as substrate assessed as AMP monomers and AT...More data for this Ligand-Target Pair
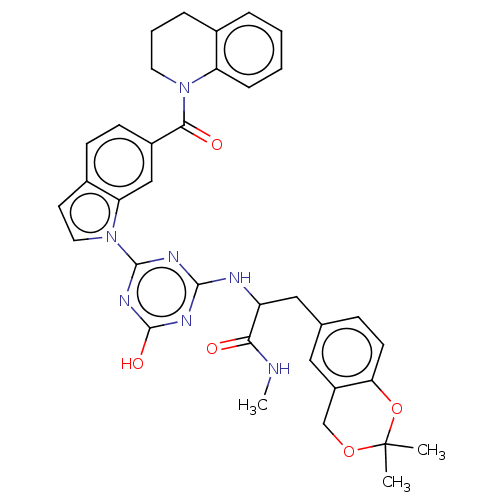
Affinity DataIC50: 2nMAssay Description:Competitive inhibition of N-terminal His-tagged human recombinant sEH expressed in insect Sf21 cells using Epoxy Fluor 7 as substrate preincubated fo...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Competitive inhibition of N-terminal His-tagged human recombinant sEH expressed in insect Sf21 cells using Epoxy Fluor 7 as substrate preincubated fo...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
University of Utah
Curated by ChEMBL
University of Utah
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of chicken Src kinase domain using AEEEIYGEFAKKK as substrate by continuous spectrophotometric assayMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
University of Utah
Curated by ChEMBL
University of Utah
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of chicken Src kinase domain using AEEEIYGEFAKKK as substrate by continuous spectrophotometric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human Wip1 (2 to 420 residues) expressed in baculovirus-infected insect SF9 cells assessed as fluorescein diphosphate hydrolysis after ...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human Wip1 (2 to 420 residues) expressed in baculovirus-infected insect SF9 cells assessed as fluorescein diphosphate hydrolysis after ...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
University of Utah
Curated by ChEMBL
University of Utah
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of p38 MAPK (unknown origin)More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 11/12/13/14(Homo sapiens (Human))
University of Utah
Curated by ChEMBL
University of Utah
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of p38 MAPK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Displacement of GSK215 from full length PAD4 (unknown origin) after 50 mins by fluorescence polarization assay in absence of calciumMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of IDE (unknown origin) assessed as cleavage of Mca-RPPGFSAFK(Dnp)-OH by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Displacement of GSK215 from full length PAD4 (unknown origin) after 50 mins by fluorescence polarization assay in absence of calciumMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of IDE (unknown origin) assessed as cleavage of Mca-RPPGFSAFK(Dnp)-OH by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:Inhibition of aurora A kinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:Inhibition of aurora A kinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataKd: 930nMAssay Description:Binding affinity to human Bcl-xL after 1 hr by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKd: 4.20E+3nMAssay Description:Binding affinity to human IL-2 measured over 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKd: 930nMAssay Description:Binding affinity to human Bcl-xL after 1 hr by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKd: 4.20E+3nMAssay Description:Binding affinity to human IL-2 measured over 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair