Found 32 hits of Enzyme Inhibition Constant Data
Found 32 hits of Enzyme Inhibition Constant Data
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
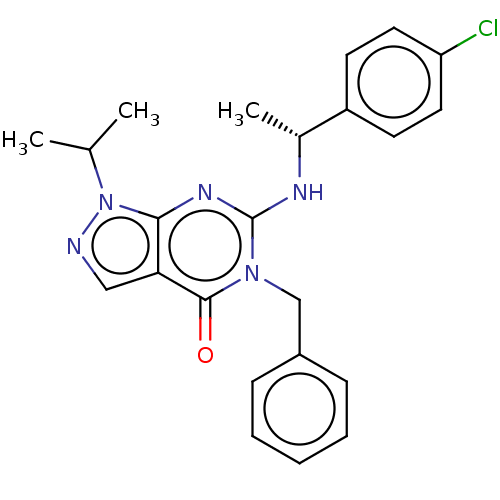
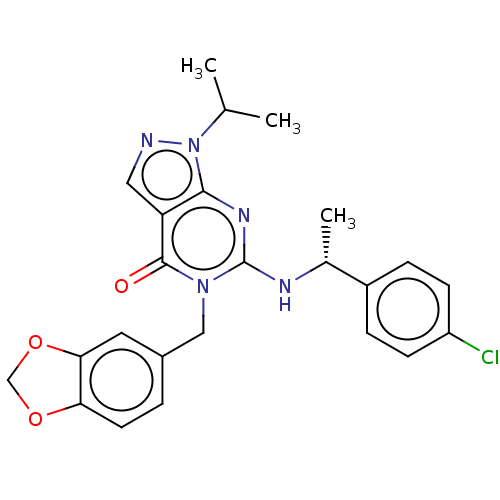
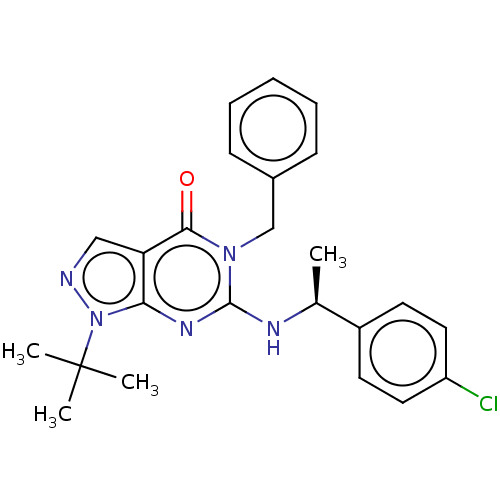
Affinity DataIC50: 0.900nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
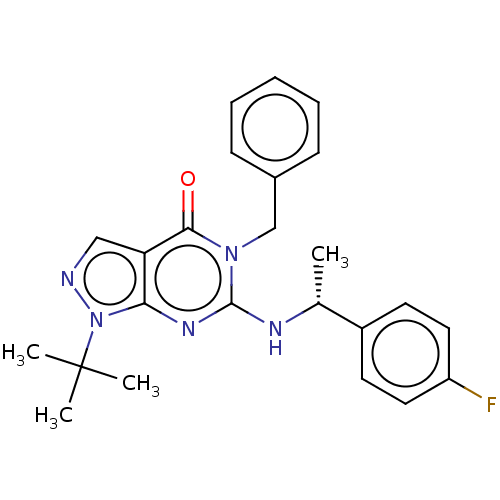
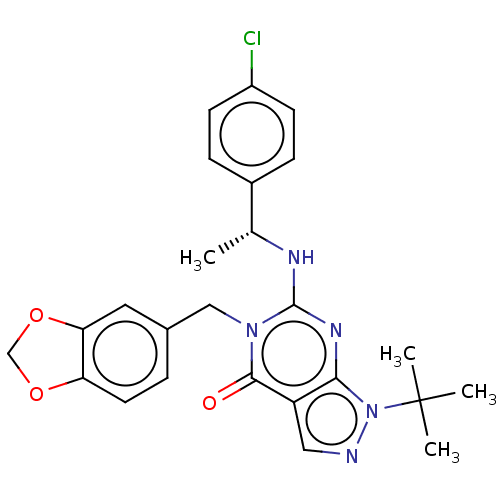
Affinity DataIC50: 1.5nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
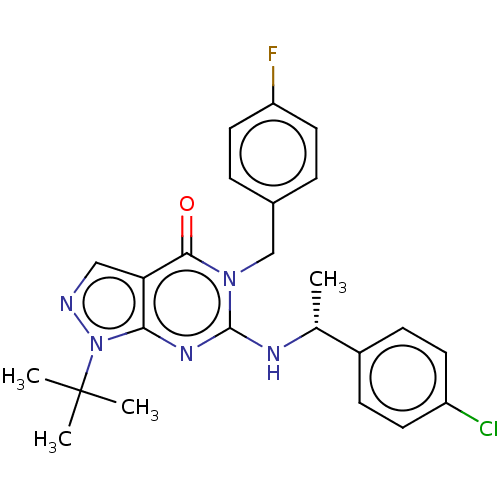
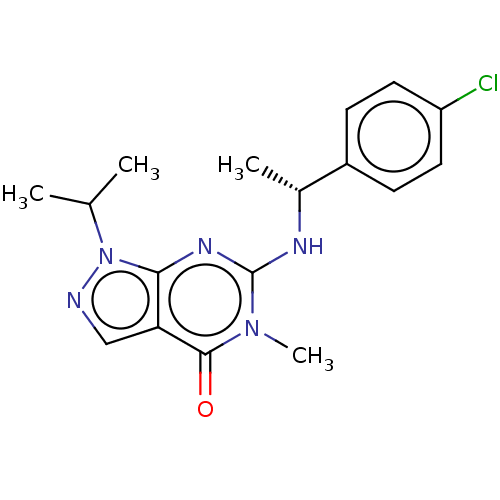
Affinity DataIC50: 2.60nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
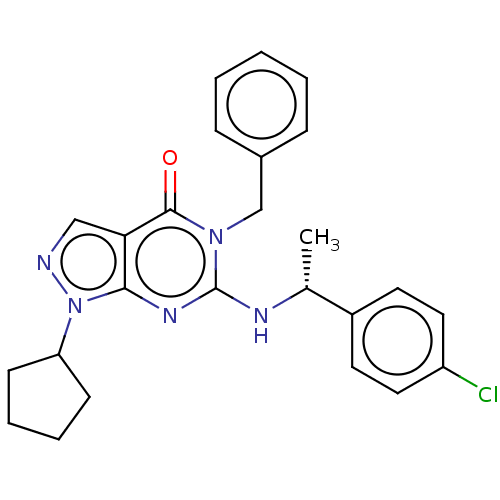
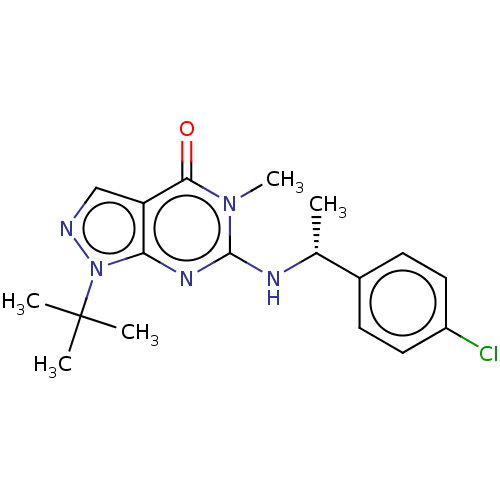
Affinity DataIC50: 2.90nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
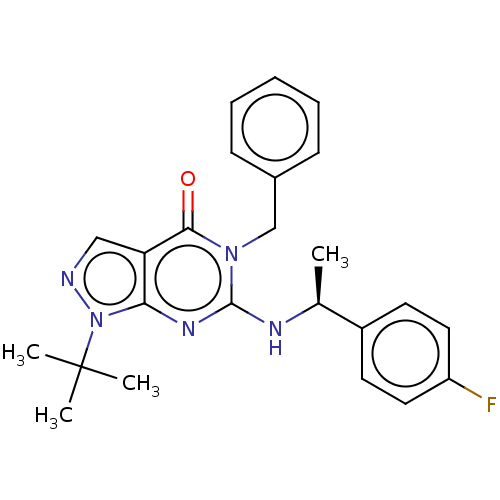
Affinity DataIC50: 2.90nMAssay Description:Inhibition of PDE1B (146 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 4.70nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of PDE1B (146 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 57nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
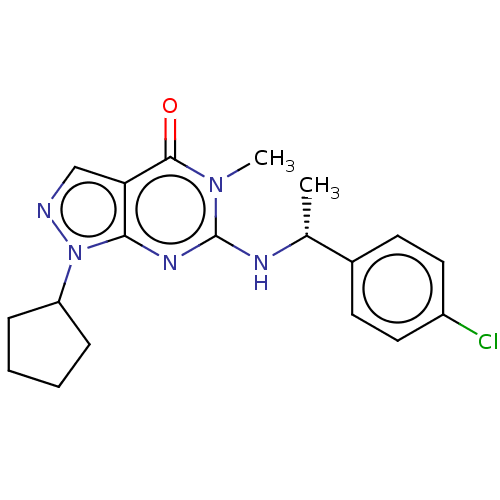
Affinity DataIC50: 58nMAssay Description:Inhibition of PDE9A (181 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of PDE1B (146 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 157nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 245nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 258nMAssay Description:Inhibition of PDE1C2 (147 to 531 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 370nMAssay Description:Inhibition of PDE5A1 (535 to 860 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method r...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 699nMAssay Description:Inhibition of PDE4D2 (86 to 413 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method re...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 1.77E+3nMAssay Description:Inhibition of PDE10A (449 to 770 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method r...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 2.02E+3nMAssay Description:Inhibition of PDE8A1 (480 to 820 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method r...More data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
TargetCone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha'(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PDE6C (1 to 858 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method rela...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PDE9A (181 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetcGMP-dependent 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PDE2A (580 to 919 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method re...More data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PDE3A (679 to 1087 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method r...More data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PDE7A1 (130 to 482 residues) (unknown origin) using [3H]-cAMP substrate incubated for 15 mins by liquid scintillation counting method r...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PDE9A2 (181 to 506 residues) (unknown origin) using [3H]-cGMP substrate incubated for 15 mins by liquid scintillation counting method r...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Sun Yat-sen University
Curated by ChEMBL
Sun Yat-sen University
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human CYP3A4 using testosterone as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human CYP3A4 using midazolam as substrateMore data for this Ligand-Target Pair