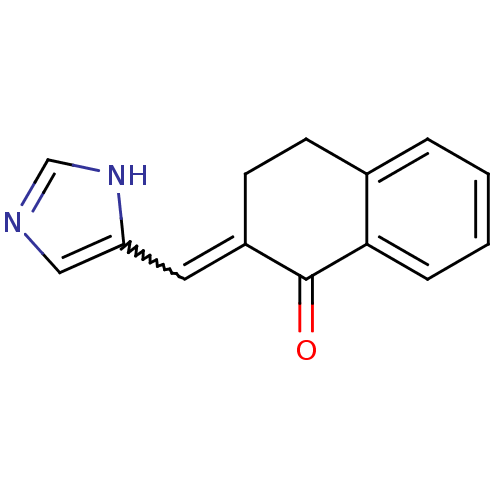
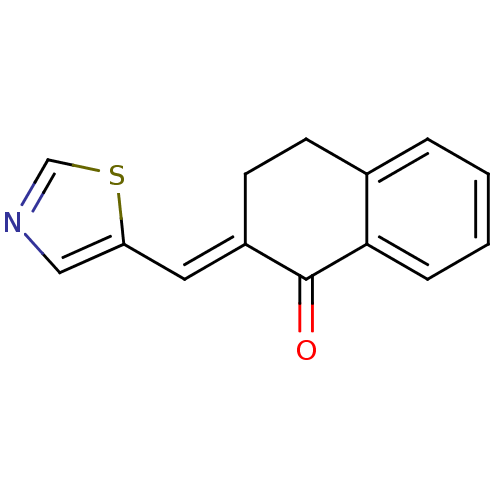
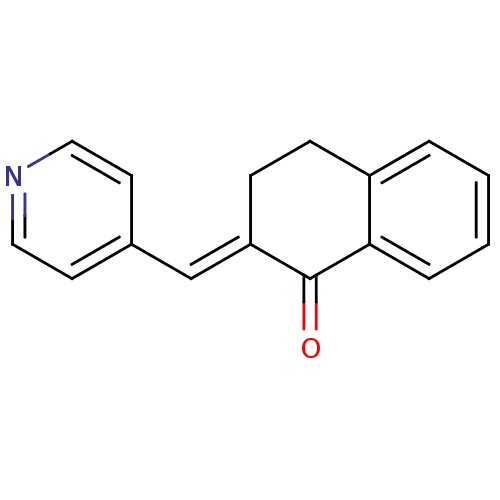
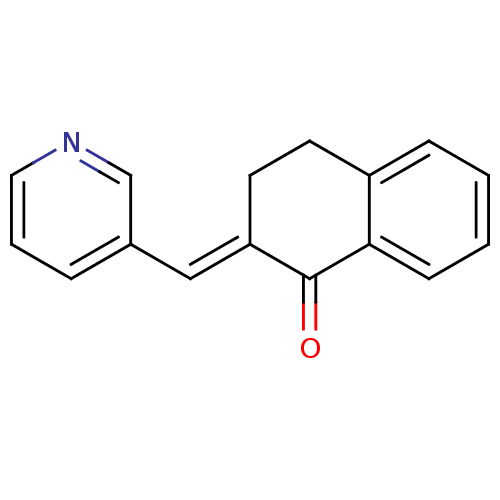
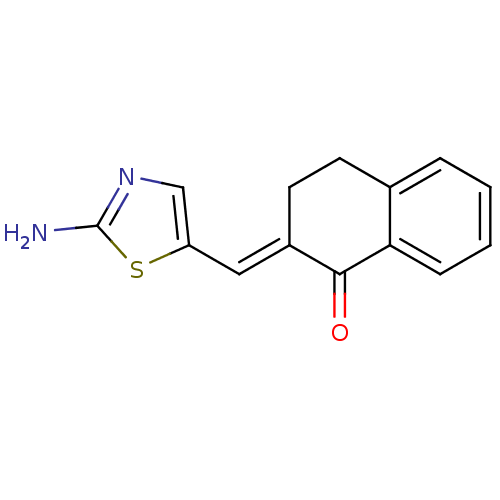
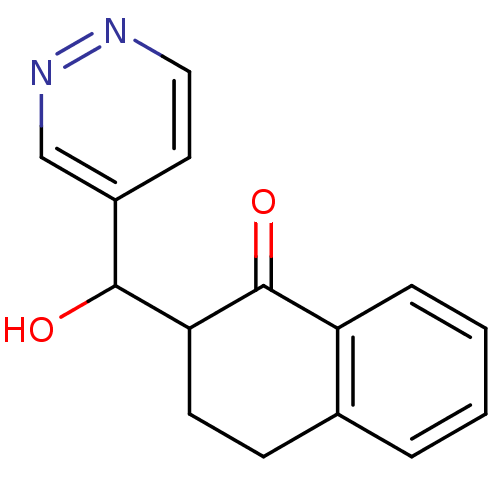
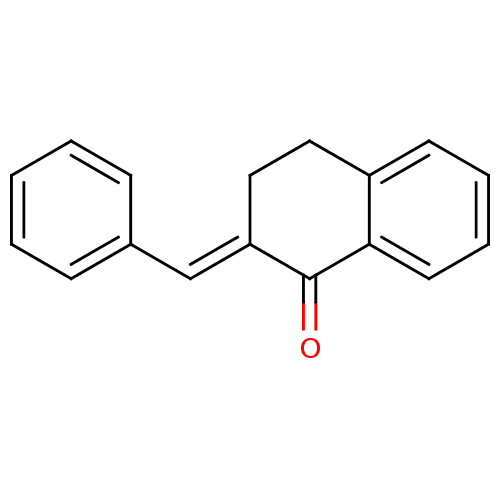
Found 43 hits of Enzyme Inhibition Constant Data
Found 43 hits of Enzyme Inhibition Constant Data
Affinity DataIC50: 170nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 370nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+3nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 9.20E+3nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 2.10E+4nMAssay Description:In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 3.10E+4nMAssay Description:In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 3.60E+4nMAssay Description:In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 3.70E+4nMAssay Description:In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 4.30E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 4.80E+4nMAssay Description:In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+4nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 5.50E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 7.30E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 7.40E+4nMAssay Description:In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 8.70E+4nMAssay Description:In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 9.00E+4nMAssay Description:In vitro inhibitory activity against Steroidgenic Cytochrome P450 17 alpha using rat testicular microsomes.More data for this Ligand-Target Pair
TargetCytochrome P450 11B2, mitochondrial(Homo sapiens (Human))
Universität de Saarlandes
Curated by ChEMBL
Universität de Saarlandes
Curated by ChEMBL
Affinity DataIC50: 9.00E+4nMAssay Description:Inhibitory activity against Steroidgenic Cytochrome P450 C18 using Bovine adrenal mitochondria.More data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:In vitro inhibitory activity against Cytochrome P450 19A1 using human placental microsomes.More data for this Ligand-Target Pair