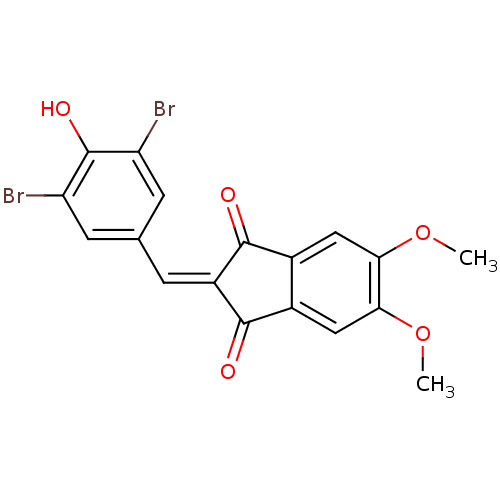
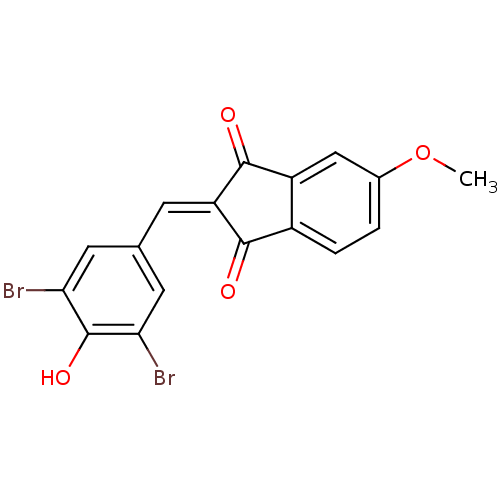
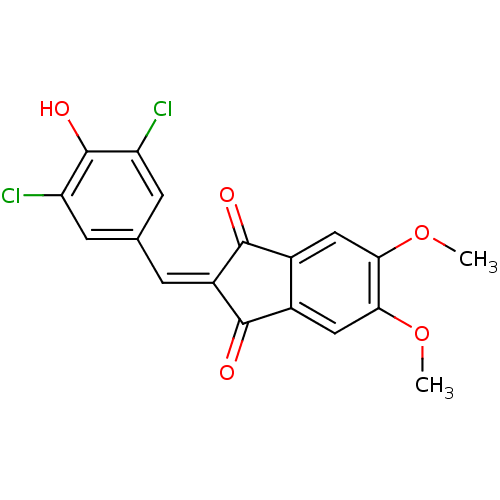
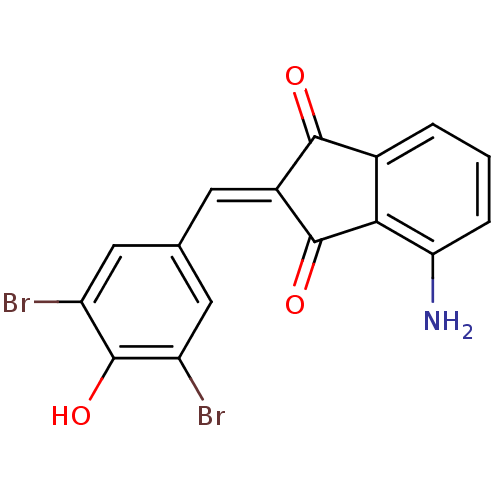
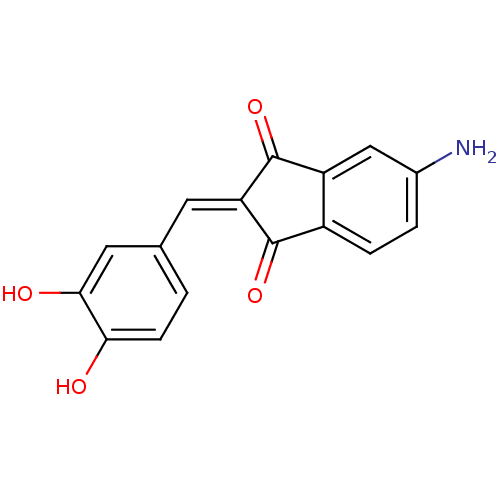
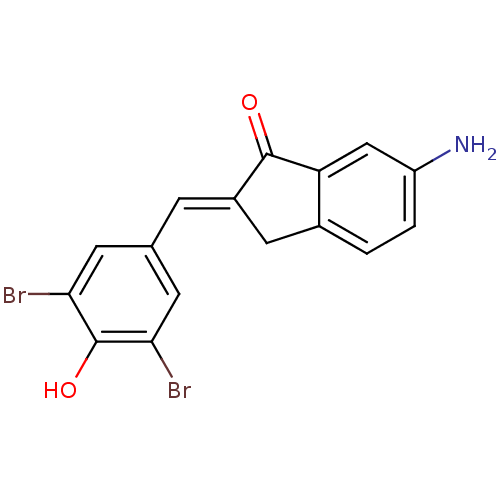
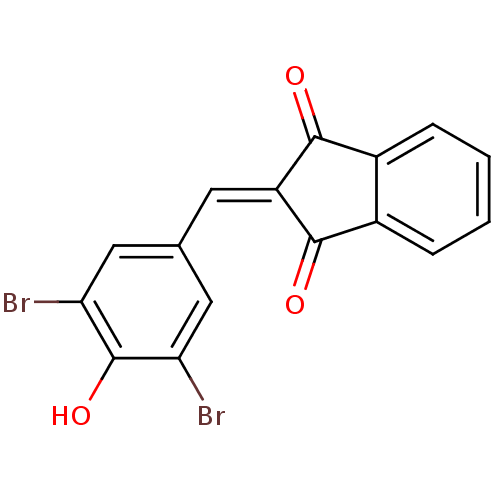
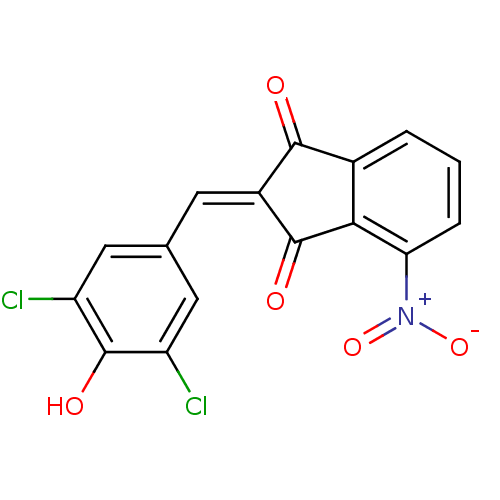
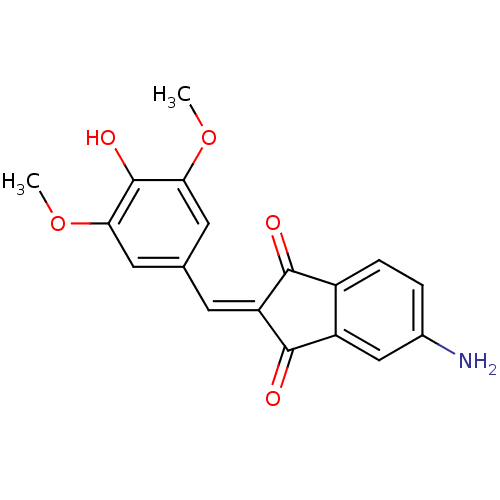
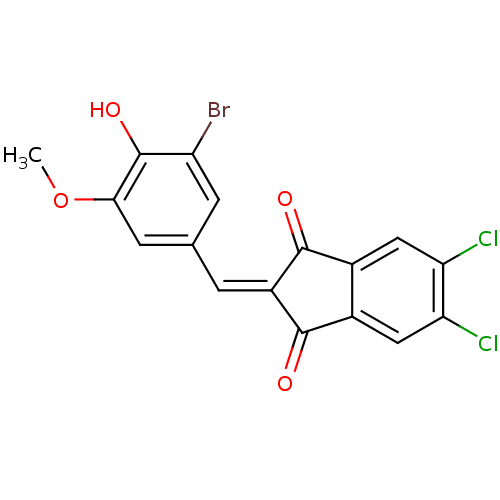
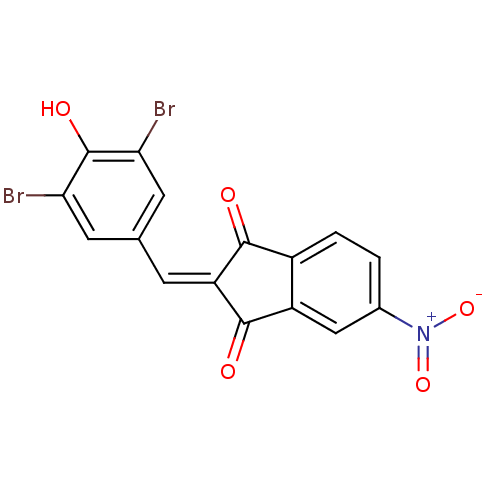
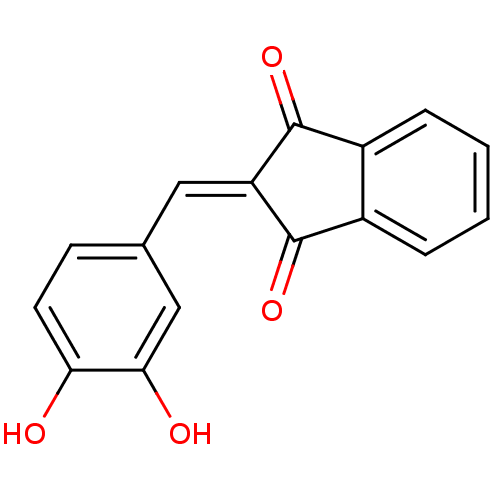
Found 28 hits of Enzyme Inhibition Constant Data
Found 28 hits of Enzyme Inhibition Constant Data
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 102nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 144nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 310nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of Src tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 470nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 710nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 740nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 880nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of Src tyrosine kinaseMore data for this Ligand-Target Pair
TargetcAMP-dependent protein kinase catalytic subunit alpha/beta/gamma(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of Protein Kinase A (PKA)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetcAMP-dependent protein kinase catalytic subunit alpha/beta/gamma(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of Protein Kinase A (PKA)More data for this Ligand-Target Pair
TargetcAMP-dependent protein kinase catalytic subunit alpha/beta/gamma(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Protein Kinase A (PKA)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
R. W. Johnson Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of Src tyrosine kinaseMore data for this Ligand-Target Pair