Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
Ligand
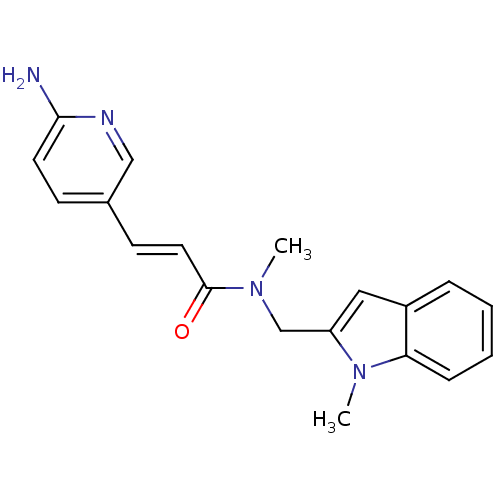
BDBM8720
Substrate
Crotonoyl-ACP
Meas. Tech.
Enzyme Inhibition Assay
Temperature
303.15±n/a K
IC50
4200±n/a nM
Comments
extracted
Citation
 Miller, WH; Seefeld, MA; Newlander, KA; Uzinskas, IN; Burgess, WJ; Heerding, DA; Yuan, CC; Head, MS; Payne, DJ; Rittenhouse, SF; Moore, TD; Pearson, SC; Berry, V; DeWolf, WE; Keller, PM; Polizzi, BJ; Qiu, X; Janson, CA; Huffman, WF Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI). J Med Chem 45:3246-56 (2002) [PubMed] Article
Miller, WH; Seefeld, MA; Newlander, KA; Uzinskas, IN; Burgess, WJ; Heerding, DA; Yuan, CC; Head, MS; Payne, DJ; Rittenhouse, SF; Moore, TD; Pearson, SC; Berry, V; DeWolf, WE; Keller, PM; Polizzi, BJ; Qiu, X; Janson, CA; Huffman, WF Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI). J Med Chem 45:3246-56 (2002) [PubMed] ArticleMore Info.:
Target
Name:
Enoyl-[acyl-carrier-protein] reductase [NADH] FabI
Synonyms:
Enoyl-ACP Reductase (FabI) | FABI_HAEIN | NADH-dependent enoyl-ACP reductase | envM | fabI
Type:
Enzyme
Mol. Mass.:
28115.25
Organism:
Haemophilus influenzae
Description:
P44432
Residue:
262
Sequence:
MGFLTGKRILVTGLASNRSIAYGIAKSMKEQGAELAFTYLNDKLQPRVEEFAKEFGSDIVLPLDVATDESIQNCFAELSKRWDKFDGFIHAIAFAPGDQLDGDYVNAATREGYRIAHDISAYSFVAMAQAARPYLNPNAALLTLSYLGAERAIPNYNVMCLAKASLEAATRVMAADLGKEGIRVNAISAGPIRTLAASGIKNFKKMLSTFEKTAALRRTVTIEDVGNSAAFLCSDLASGITGEIVHVDAGFSITAMGELGEE
Inhibitor
Name:
BDBM8720
Synonyms:
(2E)-3-(6-aminopyridin-3-yl)-N-methyl-N-[(1-methyl-1H-indol-2-yl)methyl]prop-2-enamide | 3-(6-Aminopyridin-3-yl)-N-methyl-N-[(1-methyl-1H-indol-2-yl)methyl]acrylamide | aminopyridine 4 | aminopyridine deriv. 9
Type:
Small organic molecule
Emp. Form.:
C19H20N4O
Mol. Mass.:
320.3883
SMILES:
CN(Cc1cc2ccccc2n1C)C(=O)\C=C\c1ccc(N)nc1
Substrate
Name:
Crotonoyl-ACP
Synonyms:
n/a
Type:
Other Protein Type
Mol. Mass.:
358.43
Organism:
n/a
Description:
1. NAD(P)H is its co-substrate. 2. Crotonoyl-ACP was synthesized using ACP synthase to catalyse the addition of a crotonoyl group from crotonoyl-CoA to apo-ACP
Residue:
3
Sequence:
NA