Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Carbonic anhydrase 2
Ligand
BDBM11331
Substrate
BDBM11326
Meas. Tech.
Carbonic Anhydrase Enzyme Inhibition Assay
Ki
120±n/a nM
Citation
 Scozzafava, A; Supuran, CT Carbonic anhydrase and matrix metalloproteinase inhibitors: sulfonylated amino acid hydroxamates with MMP inhibitory properties act as efficient inhibitors of CA isozymes I, II, and IV, and N-hydroxysulfonamides inhibit both these zinc enzymes. J Med Chem 43:3677-87 (2000) [PubMed] Article
Scozzafava, A; Supuran, CT Carbonic anhydrase and matrix metalloproteinase inhibitors: sulfonylated amino acid hydroxamates with MMP inhibitory properties act as efficient inhibitors of CA isozymes I, II, and IV, and N-hydroxysulfonamides inhibit both these zinc enzymes. J Med Chem 43:3677-87 (2000) [PubMed] ArticleMore Info.:
Target
Name:
Carbonic anhydrase 2
Synonyms:
CA-II | CA2 | CAC | CAH2_HUMAN | Carbonate dehydratase II | Carbonic anhydrase 2 (CA II) | Carbonic anhydrase 2 (CA-II) | Carbonic anhydrase 2 (Recombinant CA II) | Carbonic anhydrase C | Carbonic anhydrase II (CA II) | Carbonic anhydrase II (CA-II) | Carbonic anhydrase II (CAII) | Carbonic anhydrase II (hCA II) | Carbonic anhydrase isoenzyme II (hCA II)
Type:
Enzyme
Mol. Mass.:
29250.71
Organism:
Homo sapiens (Human)
Description:
P00918
Residue:
260
Sequence:
MSHHWGYGKHNGPEHWHKDFPIAKGERQSPVDIDTHTAKYDPSLKPLSVSYDQATSLRILNNGHAFNVEFDDSQDKAVLKGGPLDGTYRLIQFHFHWGSLDGQGSEHTVDKKKYAAELHLVHWNTKYGDFGKAVQQPDGLAVLGIFLKVGSAKPGLQKVVDVLDSIKTKGKSADFTNFDPRGLLPESLDYWTYPGSLTTPPLLECVTWIVLKEPISVSSEQVLKFRKLNFNGEGEPEELMVDNWRPAQPLKNRQIKASFK
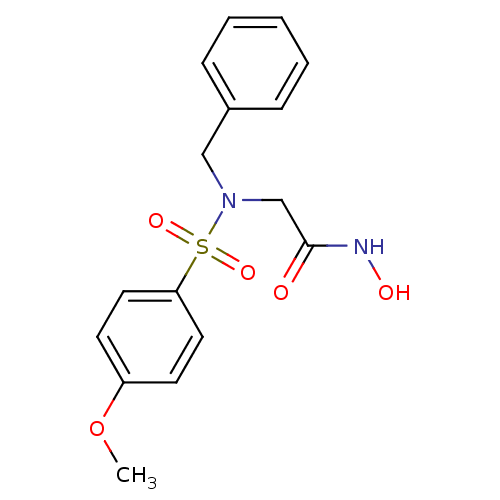
Inhibitor
Name:
BDBM11331
Synonyms:
2-[benzyl(4-methoxybenzene)sulfonamido]-N-hydroxyacetamide | CGS 27023A Analog 22 | Hydroxamate 12 | US20230357139, Compound S
Type:
Small organic molecule
Emp. Form.:
C16H18N2O5S
Mol. Mass.:
350.39
SMILES:
COc1ccc(cc1)S(=O)(=O)N(CC(=O)NO)Cc1ccccc1