Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Peroxisome proliferator-activated receptor gamma
Ligand
BDBM50109547
Substrate
n/a
Meas. Tech.
ChEMBL_220893 (CHEMBL824648)
EC50
170±n/a nM
Citation
 Sauerberg, P; Pettersson, I; Jeppesen, L; Bury, PS; Mogensen, JP; Wassermann, K; Brand, CL; Sturis, J; Wöldike, HF; Fleckner, J; Andersen, AS; Mortensen, SB; Svensson, LA; Rasmussen, HB; Lehmann, SV; Polivka, Z; Sindelar, K; Panajotova, V; Ynddal, L; Wulff, EM Novel tricyclic-alpha-alkyloxyphenylpropionic acids: dual PPARalpha/gamma agonists with hypolipidemic and antidiabetic activity. J Med Chem 45:789-804 (2002) [PubMed]
Sauerberg, P; Pettersson, I; Jeppesen, L; Bury, PS; Mogensen, JP; Wassermann, K; Brand, CL; Sturis, J; Wöldike, HF; Fleckner, J; Andersen, AS; Mortensen, SB; Svensson, LA; Rasmussen, HB; Lehmann, SV; Polivka, Z; Sindelar, K; Panajotova, V; Ynddal, L; Wulff, EM Novel tricyclic-alpha-alkyloxyphenylpropionic acids: dual PPARalpha/gamma agonists with hypolipidemic and antidiabetic activity. J Med Chem 45:789-804 (2002) [PubMed]More Info.:
Target
Name:
Peroxisome proliferator-activated receptor gamma
Synonyms:
NR1C3 | Nuclear receptor subfamily 1 group C member 3 | PPAR-gamma | PPARG | PPARG_HUMAN | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor gamma (PPAR gamma) | Peroxisome proliferator-activated receptor gamma (PPARG) | Peroxisome proliferator-activated receptor gamma (PPARγ) | Peroxisome proliferator-activated receptor gamma/Nuclear receptor corepressor 2 | peroxisome proliferator-activated receptor gamma isoform 2
Type:
Nuclear Receptor
Mol. Mass.:
57613.46
Organism:
Homo sapiens (Human)
Description:
P37231
Residue:
505
Sequence:
MGETLGDSPIDPESDSFTDTLSANISQEMTMVDTEMPFWPTNFGISSVDLSVMEDHSHSFDIKPFTTVDFSSISTPHYEDIPFTRTDPVVADYKYDLKLQEYQSAIKVEPASPPYYSEKTQLYNKPHEEPSNSLMAIECRVCGDKASGFHYGVHACEGCKGFFRRTIRLKLIYDRCDLNCRIHKKSRNKCQYCRFQKCLAVGMSHNAIRFGRMPQAEKEKLLAEISSDIDQLNPESADLRALAKHLYDSYIKSFPLTKAKARAILTGKTTDKSPFVIYDMNSLMMGEDKIKFKHITPLQEQSKEVAIRIFQGCQFRSVEAVQEITEYAKSIPGFVNLDLNDQVTLLKYGVHEIIYTMLASLMNKDGVLISEGQGFMTREFLKSLRKPFGDFMEPKFEFAVKFNALELDDSDLAIFIAVIILSGDRPGLLNVKPIEDIQDNLLQALELQLKLNHPESSQLFAKLLQKMTDLRQIVTEHVQLLQVIKKTETDMSLHPLLQEIYKDLY
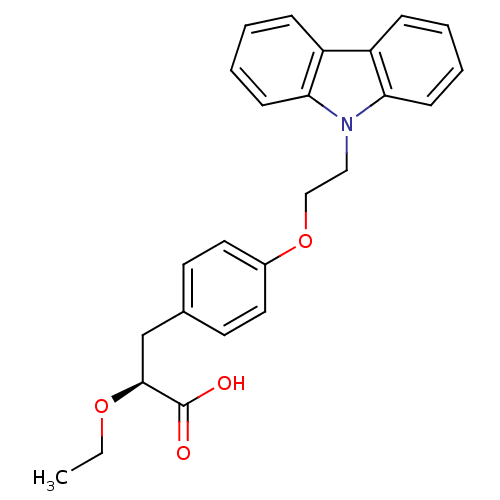
Inhibitor
Name:
BDBM50109547
Synonyms:
(S)-3-(4-(2-CARBAZOL-9-YL-ETHOXY)-PHENYL)-2-ETHOXY-PROPIONIC ACID | (S)-3-[4-(2-Carbazol-9-yl-ethoxy)-phenyl]-2-ethoxy-propionic acid | 3-[4-(2-Carbazol-9-yl-ethoxy)-phenyl]-2-ethoxy-propionic acid | CHEMBL86658
Type:
Small organic molecule
Emp. Form.:
C25H25NO4
Mol. Mass.:
403.4703
SMILES:
CCO[C@@H](Cc1ccc(OCCn2c3ccccc3c3ccccc23)cc1)C(O)=O