Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
Ligand
BDBM50602499
Substrate
n/a
Meas. Tech.
ChEMBL_2241054 (CHEMBL5155264)
IC50
2.0±n/a nM
Citation
 Mata, G; Miles, DH; Drew, SL; Fournier, J; Lawson, KV; Mailyan, AK; Sharif, EU; Yan, X; Beatty, JW; Banuelos, J; Chen, J; Ginn, E; Chen, A; Gerrick, KY; Pham, AT; Wong, K; Soni, D; Dhanota, P; Shaqfeh, SG; Meleza, C; Narasappa, N; Singh, H; Zhao, X; Jin, L; Schindler, U; Walters, MJ; Young, SW; Walker, NP; Leleti, MR; Powers, JP; Jeffrey, JL Design, Synthesis, and Structure-Activity Relationship Optimization of Pyrazolopyrimidine Amide Inhibitors of Phosphoinositide 3-Kinase ? (PI3K?). J Med Chem 65:1418-1444 (2022) [PubMed] Article
Mata, G; Miles, DH; Drew, SL; Fournier, J; Lawson, KV; Mailyan, AK; Sharif, EU; Yan, X; Beatty, JW; Banuelos, J; Chen, J; Ginn, E; Chen, A; Gerrick, KY; Pham, AT; Wong, K; Soni, D; Dhanota, P; Shaqfeh, SG; Meleza, C; Narasappa, N; Singh, H; Zhao, X; Jin, L; Schindler, U; Walters, MJ; Young, SW; Walker, NP; Leleti, MR; Powers, JP; Jeffrey, JL Design, Synthesis, and Structure-Activity Relationship Optimization of Pyrazolopyrimidine Amide Inhibitors of Phosphoinositide 3-Kinase ? (PI3K?). J Med Chem 65:1418-1444 (2022) [PubMed] ArticleMore Info.:
Target
Name:
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
Synonyms:
PI3-kinase p110 subunit gamma | PI3-kinase subunit p120-gamma | PI3Kgamma | PIK3CG | PK3CG_HUMAN | Phosphatidylinositol 4,5-biphosphate 3-kinase catalytic subunit gamma (PIK3CG) | Phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase 110 kDa catalytic subunit gamma (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma (PI3Kgamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3Kgamma) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | Phosphoinositide 3-Kinase (PI3K), gamma Chain A | Phosphoinositide 3-kinases gamma (PI3K gamma) | Phosphoinositide-3-kinase (PI3K gamma) | p120-PI3K
Type:
Enzyme Subunit
Mol. Mass.:
126470.30
Organism:
Homo sapiens (Human)
Description:
P48736
Residue:
1102
Sequence:
MELENYKQPVVLREDNCRRRRRMKPRSAAASLSSMELIPIEFVLPTSQRKCKSPETALLHVAGHGNVEQMKAQVWLRALETSVAADFYHRLGPHHFLLLYQKKGQWYEIYDKYQVVQTLDCLRYWKATHRSPGQIHLVQRHPPSEESQAFQRQLTALIGYDVTDVSNVHDDELEFTRRGLVTPRMAEVASRDPKLYAMHPWVTSKPLPEYLWKKIANNCIFIVIHRSTTSQTIKVSPDDTPGAILQSFFTKMAKKKSLMDIPESQSEQDFVLRVCGRDEYLVGETPIKNFQWVRHCLKNGEEIHVVLDTPPDPALDEVRKEEWPLVDDCTGVTGYHEQLTIHGKDHESVFTVSLWDCDRKFRVKIRGIDIPVLPRNTDLTVFVEANIQHGQQVLCQRRTSPKPFTEEVLWNVWLEFSIKIKDLPKGALLNLQIYCGKAPALSSKASAESPSSESKGKVQLLYYVNLLLIDHRFLLRRGEYVLHMWQISGKGEDQGSFNADKLTSATNPDKENSMSISILLDNYCHPIALPKHQPTPDPEGDRVRAEMPNQLRKQLEAIIATDPLNPLTAEDKELLWHFRYESLKHPKAYPKLFSSVKWGQQEIVAKTYQLLARREVWDQSALDVGLTMQLLDCNFSDENVRAIAVQKLESLEDDDVLHYLLQLVQAVKFEPYHDSALARFLLKRGLRNKRIGHFLFWFLRSEIAQSRHYQQRFAVILEAYLRGCGTAMLHDFTQQVQVIEMLQKVTLDIKSLSAEKYDVSSQVISQLKQKLENLQNSQLPESFRVPYDPGLKAGALAIEKCKVMASKKKPLWLEFKCADPTALSNETIGIIFKHGDDLRQDMLILQILRIMESIWETESLDLCLLPYGCISTGDKIGMIEIVKDATTIAKIQQSTVGNTGAFKDEVLNHWLKEKSPTEEKFQAAVERFVYSCAGYCVATFVLGIGDRHNDNIMITETGNLFHIDFGHILGNYKSFLGINKERVPFVLTPDFLFVMGTSGKKTSPHFQKFQDICVKAYLALRHHTNLLIILFSMMLMTGMPQLTSKEDIEYIRDALTVGKNEEDAKKYFLDQIEVCRDKGWTVQFNWFLHLVLGIKQGEKHSA
Inhibitor
Name:
BDBM50602499
Synonyms:
CHEMBL5178231
Type:
Small organic molecule
Emp. Form.:
C27H33N7O2
Mol. Mass.:
487.5966
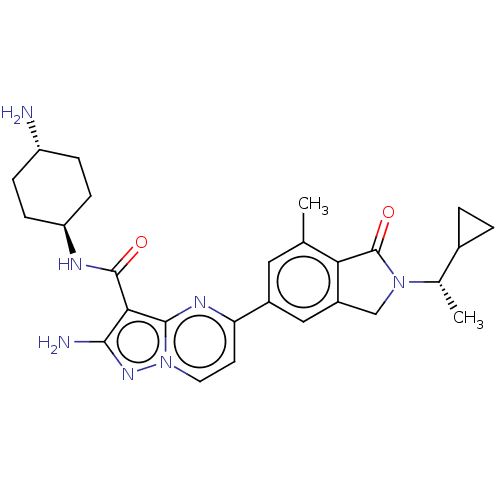
SMILES:
C[C@@H](C1CC1)N1Cc2cc(cc(C)c2C1=O)-c1ccn2nc(N)c(C(=O)N[C@H]3CC[C@H](N)CC3)c2n1 |r,wU:1.1,30.33,wD:27.29,(7.27,2.89,;6.5,1.55,;7.27,.22,;7.27,-1.32,;8.6,-.55,;4.96,1.55,;4.06,2.8,;2.59,2.32,;1.26,3.09,;-.07,2.32,;-.07,.78,;1.26,.01,;1.26,-1.53,;2.59,.78,;4.06,.31,;4.45,-1.18,;-1.41,3.09,;-1.41,4.63,;-2.73,5.39,;-4.06,4.63,;-5.52,5.1,;-6.43,3.86,;-7.97,3.86,;-5.52,2.61,;-6.29,1.28,;-7.83,1.28,;-5.52,-.06,;-6.29,-1.39,;-7.83,-1.39,;-8.6,-2.72,;-7.83,-4.06,;-8.6,-5.39,;-6.29,-4.06,;-5.52,-2.72,;-4.06,3.09,;-2.73,2.33,)|