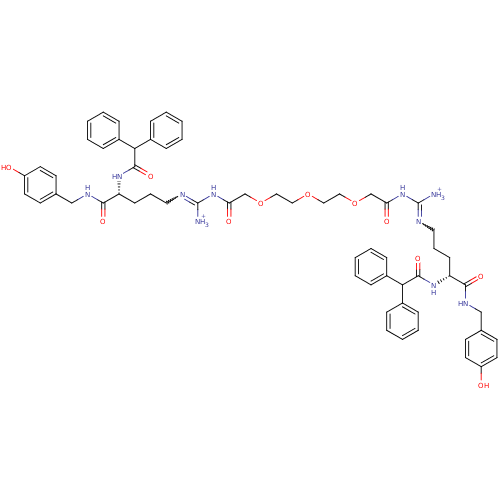
Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neuropeptide Y receptor type 1
Ligand
BDBM50273946
Substrate
n/a
Meas. Tech.
ChEMBL_537978 (CHEMBL1032434)
Ki
6.1±n/a nM
Citation
 Weiss, S; Keller, M; Bernhardt, G; Buschauer, A; König, B Modular synthesis of non-peptidic bivalent NPY Y1 receptor antagonists. Bioorg Med Chem 16:9858-66 (2008) [PubMed] Article
Weiss, S; Keller, M; Bernhardt, G; Buschauer, A; König, B Modular synthesis of non-peptidic bivalent NPY Y1 receptor antagonists. Bioorg Med Chem 16:9858-66 (2008) [PubMed] ArticleMore Info.:
Target
Name:
Neuropeptide Y receptor type 1
Synonyms:
NPY-Y1 | NPY1-R | NPY1R | NPY1R_HUMAN | NPYR | NPYY1 | neuropeptide Y receptor Y1
Type:
Enzyme Catalytic Domain
Mol. Mass.:
44399.07
Organism:
Homo sapiens (Human)
Description:
NPY-Y1 NPY1R HUMAN::P25929
Residue:
384
Sequence:
MNSTLFSQVENHSVHSNFSEKNAQLLAFENDDCHLPLAMIFTLALAYGAVIILGVSGNLALIIIILKQKEMRNVTNILIVNLSFSDLLVAIMCLPFTFVYTLMDHWVFGEAMCKLNPFVQCVSITVSIFSLVLIAVERHQLIINPRGWRPNNRHAYVGIAVIWVLAVASSLPFLIYQVMTDEPFQNVTLDAYKDKYVCFDQFPSDSHRLSYTTLLLVLQYFGPLCFIFICYFKIYIRLKRRNNMMDKMRDNKYRSSETKRINIMLLSIVVAFAVCWLPLTIFNTVFDWNHQIIATCNHNLLFLLCHLTAMISTCVNPIFYGFLNKNFQRDLQFFFNFCDFRSRDDDYETIAMSTMHTDVSKTSLKQASPVAFKKINNNDDNEKI
Inhibitor
Name:
BDBM50273946
Synonyms:
((14Z,20R)-15-Ammonio-1-({(4R)-4-[(diphenylacetyl)-amino]-5-[(4-hydroxybenzyl)amino]-5-oxopentyl}amino)-20-{[(4-hydroxybenzyl)amino]carbonyl}-3,13,22-trioxo-23,23-diphenyl-5,8,11-trioxa-2,14,16,21-tetraazatricos-14-en-1-ylidene)ammonium bis(trifluoroacetate) | CHEMBL450031
Type:
Small organic molecule
Emp. Form.:
C62H74N10O11
Mol. Mass.:
1135.3103
SMILES:
[NH3+]C(NC(=O)COCCOCCOCC(=O)NC([NH3+])=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1)=NCCC[C@@H](NC(=O)C(c1ccccc1)c1ccccc1)C(=O)NCc1ccc(O)cc1 |r,w:51.54,19.19|