Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Nitric oxide synthase, endothelial
Ligand
BDBM50365340
Substrate
n/a
Meas. Tech.
ChEMBL_806490 (CHEMBL1959623)
IC50
220000±n/a nM
Citation
 Annedi, SC; Ramnauth, J; Maddaford, SP; Renton, P; Rakhit, S; Mladenova, G; Dove, P; Silverman, S; Andrews, JS; Felice, MD; Porreca, F Discovery of cis-N-(1-(4-(methylamino)cyclohexyl)indolin-6-yl)thiophene-2-carboximidamide: a 1,6-disubstituted indoline derivative as a highly selective inhibitor of human neuronal nitric oxide synthase (nNOS) without any cardiovascular liabilities. J Med Chem 55:943-55 (2012) [PubMed] Article
Annedi, SC; Ramnauth, J; Maddaford, SP; Renton, P; Rakhit, S; Mladenova, G; Dove, P; Silverman, S; Andrews, JS; Felice, MD; Porreca, F Discovery of cis-N-(1-(4-(methylamino)cyclohexyl)indolin-6-yl)thiophene-2-carboximidamide: a 1,6-disubstituted indoline derivative as a highly selective inhibitor of human neuronal nitric oxide synthase (nNOS) without any cardiovascular liabilities. J Med Chem 55:943-55 (2012) [PubMed] ArticleMore Info.:
Target
Name:
Nitric oxide synthase, endothelial
Synonyms:
Constitutive NOS | EC-NOS | Endothelial NOS | Endothelial nitric oxide synthase | NOS type III | NOS3 | NOS3_HUMAN | NOSIII | Nitric oxide synthase (inducible and endothelial) | Nitric oxide synthase, endothelial (eNOS) | Nitric-oxide synthase (endothelial and brain) | cNOS | eNOS
Type:
Enzyme Catalytic Domain
Mol. Mass.:
133297.84
Organism:
Homo sapiens (Human)
Description:
P29474
Residue:
1203
Sequence:
MGNLKSVAQEPGPPCGLGLGLGLGLCGKQGPATPAPEPSRAPASLLPPAPEHSPPSSPLTQPPEGPKFPRVKNWEVGSITYDTLSAQAQQDGPCTPRRCLGSLVFPRKLQGRPSPGPPAPEQLLSQARDFINQYYSSIKRSGSQAHEQRLQEVEAEVAATGTYQLRESELVFGAKQAWRNAPRCVGRIQWGKLQVFDARDCRSAQEMFTYICNHIKYATNRGNLRSAITVFPQRCPGRGDFRIWNSQLVRYAGYRQQDGSVRGDPANVEITELCIQHGWTPGNGRFDVLPLLLQAPDDPPELFLLPPELVLEVPLEHPTLEWFAALGLRWYALPAVSNMLLEIGGLEFPAAPFSGWYMSTEIGTRNLCDPHRYNILEDVAVCMDLDTRTTSSLWKDKAAVEINVAVLHSYQLAKVTIVDHHAATASFMKHLENEQKARGGCPADWAWIVPPISGSLTPVFHQEMVNYFLSPAFRYQPDPWKGSAAKGTGITRKKTFKEVANAVKISASLMGTVMAKRVKATILYGSETGRAQSYAQQLGRLFRKAFDPRVLCMDEYDVVSLEHETLVLVVTSTFGNGDPPENGESFAAALMEMSGPYNSSPRPEQHKSYKIRFNSISCSDPLVSSWRRKRKESSNTDSAGALGTLRFCVFGLGSRAYPHFCAFARAVDTRLEELGGERLLQLGQGDELCGQEEAFRGWAQAAFQAACETFCVGEDAKAAARDIFSPKRSWKRQRYRLSAQAEGLQLLPGLIHVHRRKMFQATIRSVENLQSSKSTRATILVRLDTGGQEGLQYQPGDHIGVCPPNRPGLVEALLSRVEDPPAPTEPVAVEQLEKGSPGGPPPGWVRDPRLPPCTLRQALTFFLDITSPPSPQLLRLLSTLAEEPREQQELEALSQDPRRYEEWKWFRCPTLLEVLEQFPSVALPAPLLLTQLPLLQPRYYSVSSAPSTHPGEIHLTVAVLAYRTQDGLGPLHYGVCSTWLSQLKPGDPVPCFIRGAPSFRLPPDPSLPCILVGPGTGIAPFRGFWQERLHDIESKGLQPTPMTLVFGCRCSQLDHLYRDEVQNAQQRGVFGRVLTAFSREPDNPKTYVQDILRTELAAEVHRVLCLERGHMFVCGDVTMATNVLQTVQRILATEGDMELDEAGDVIGVLRDQQRYHEDIFGLTLRTQEVTSRIRTQSFSLQERQLRGAVPWAFDPPGSDTNSP
Inhibitor
Name:
BDBM50365340
Synonyms:
CHEMBL1956109
Type:
Small organic molecule
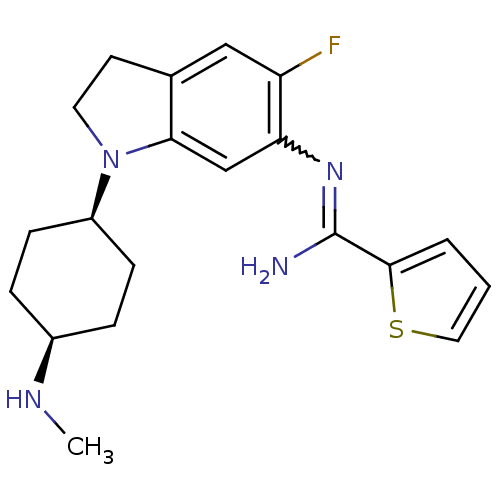
Emp. Form.:
C20H25FN4S
Mol. Mass.:
372.503
SMILES:
CN[C@H]1CC[C@H](CC1)N1CCc2cc(F)c(cc12)N=C(N)c1cccs1 |r,w:18.20,wD:5.8,2.1,(7.37,-3.67,;8.7,-2.9,;8.7,-1.36,;10.04,-.59,;10.04,.95,;8.7,1.71,;7.37,.94,;7.37,-.59,;8.71,3.26,;9.62,4.51,;8.71,5.77,;7.23,5.29,;5.9,6.05,;4.57,5.28,;3.23,6.05,;4.57,3.74,;5.9,2.97,;7.23,3.74,;3.23,2.97,;1.9,3.74,;1.9,5.28,;.57,2.97,;-.84,3.6,;-1.87,2.46,;-1.1,1.13,;.41,1.45,)|