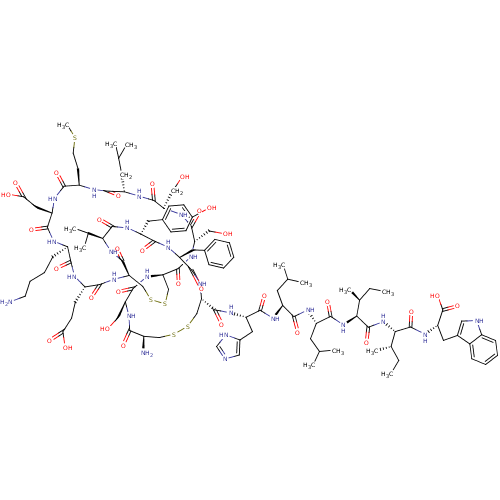
Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Endothelin-1 receptor
Ligand
BDBM50043324
Substrate
n/a
Meas. Tech.
ChEMBL_65811 (CHEMBL872385)
IC50
8.6±n/a nM
Citation
 Kikuchi, T; Kubo, K; Ohtaki, T; Suzuki, N; Asami, T; Shimamoto, N; Wakimasu, M; Fujino, M Endothelin-1 analogues substituted at both position 18 and 19: highly potent endothelin antagonists with no selectivity for either receptor subtype ETA or ETB. J Med Chem 36:4087-93 (1994) [PubMed]
Kikuchi, T; Kubo, K; Ohtaki, T; Suzuki, N; Asami, T; Shimamoto, N; Wakimasu, M; Fujino, M Endothelin-1 analogues substituted at both position 18 and 19: highly potent endothelin antagonists with no selectivity for either receptor subtype ETA or ETB. J Med Chem 36:4087-93 (1994) [PubMed]More Info.:
Target
Name:
Endothelin-1 receptor
Synonyms:
EDNRA | EDNRA_PIG | Endothelin receptor ET-A
Type:
PROTEIN
Mol. Mass.:
48707.29
Organism:
Sus scrofa
Description:
ChEMBL_65803
Residue:
427
Sequence:
METFCFRVSFWVALLGCVISDNPESHSTNLSTHVDDFTTFRGTEFSLVVTTHRPTNLALPSNGSMHNYCPQQTKITSAFKYINTVISCTIFIVGMVGNATLLRIIYQNKCMRNGPNALIASLALGDLIYVVIDLPINVFKLLAGRWPFENHDFGVFLCKLFPFLQKSSVGITVLNLCALSVDRYRAVASWSRVQGIGIPLVTAIEIVSIWILSFILAIPEAIGFVMVPFEYKGEEHKTCMLNATSKFMEFYQDVKDWWLFGFYFCMPLVCTAIFYTLMTCEMLNRRNGSLRIALSEHLKQRREVAKTVFCLVVIFALCWFPLHLSRILKKTVYDEMDKNRCELLSFLLLMDYIGINLATMNSCINPIALYFVSKKFKNCFQSCLCCCCYQSKSLMTSVPMNGTSIQWKNHEQNNHNTERSSHKDSIN
Inhibitor
Name:
BDBM50043324
Synonyms:
CHEMBL411171 | c(Cys-Ser-c(Cys-Ser-Ser-Leu-Met-Asp-Lys-Gln-Cys)-Val-Tyr-Phe-Cys)-His-Leu-Leu-Ile-Ile-Trp
Type:
Small organic molecule
Emp. Form.:
C111H165N25O30S5
Mol. Mass.:
2489.972
SMILES:
CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O