Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prothrombin
Ligand
BDBM50052425
Substrate
n/a
Meas. Tech.
ChEMBL_208545 (CHEMBL879195)
Ki
15±n/a nM
Citation
 Costanzo, MJ; Maryanoff, BE; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Kauffman, JA; Lewis, JM; Krishnan, R; Tulinsky, A Potent thrombin inhibitors that probe the S1 subsite: tripeptide transition state analogues based on a heterocycle-activated carbonyl group. J Med Chem 39:3039-43 (1996) [PubMed] Article
Costanzo, MJ; Maryanoff, BE; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Kauffman, JA; Lewis, JM; Krishnan, R; Tulinsky, A Potent thrombin inhibitors that probe the S1 subsite: tripeptide transition state analogues based on a heterocycle-activated carbonyl group. J Med Chem 39:3039-43 (1996) [PubMed] ArticleMore Info.:
Target
Name:
Prothrombin
Synonyms:
Activation peptide fragment 1 | Activation peptide fragment 2 | Coagulation factor II | F2 | Prothrombin precursor | THRB_HUMAN | Thrombin heavy chain | Thrombin light chain
Type:
Protein
Mol. Mass.:
70029.57
Organism:
Homo sapiens (Human)
Description:
P00734
Residue:
622
Sequence:
MAHVRGLQLPGCLALAALCSLVHSQHVFLAPQQARSLLQRVRRANTFLEEVRKGNLERECVEETCSYEEAFEALESSTATDVFWAKYTACETARTPRDKLAACLEGNCAEGLGTNYRGHVNITRSGIECQLWRSRYPHKPEINSTTHPGADLQENFCRNPDSSTTGPWCYTTDPTVRRQECSIPVCGQDQVTVAMTPRSEGSSVNLSPPLEQCVPDRGQQYQGRLAVTTHGLPCLAWASAQAKALSKHQDFNSAVQLVENFCRNPDGDEEGVWCYVAGKPGDFGYCDLNYCEEAVEEETGDGLDEDSDRAIEGRTATSEYQTFFNPRTFGSGEADCGLRPLFEKKSLEDKTERELLESYIDGRIVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTENDLLVRIGKHSRTRYERNIEKISMLEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHPVCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVLQVVNLPIVERPVCKDSTRIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKYGFYTHVFRLKKWIQKVIDQFGE
Inhibitor
Name:
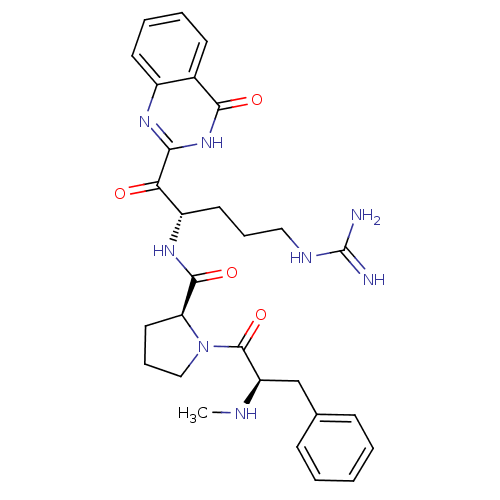
BDBM50052425
Synonyms:
(S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrrolidine-2-carboxylic acid [(S)-4-guanidino-1-(4-oxo-4,4a-dihydro-quinazoline-2-carbonyl)-butyl]-amide | CHEMBL102919 | CHEMBL165326
Type:
Small organic molecule
Emp. Form.:
C29H36N8O4
Mol. Mass.:
560.6473
SMILES:
CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)c1nc2ccccc2c(=O)[nH]1