Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Androgen receptor
Ligand
BDBM50317665
Substrate
n/a
Meas. Tech.
ChEMBL_629229 (CHEMBL1121343)
IC50
>1000±n/a nM
Citation
 Yoshino, H; Sato, H; Tachibana, K; Shiraishi, T; Nakamura, M; Ohta, M; Ishikura, N; Nagamuta, M; Onuma, E; Nakagawa, T; Arai, S; Ahn, KH; Jung, KY; Kawata, H Structure-activity relationships of bioisosteric replacement of the carboxylic acid in novel androgen receptor pure antagonists. Bioorg Med Chem 18:3159-68 (2010) [PubMed] Article
Yoshino, H; Sato, H; Tachibana, K; Shiraishi, T; Nakamura, M; Ohta, M; Ishikura, N; Nagamuta, M; Onuma, E; Nakagawa, T; Arai, S; Ahn, KH; Jung, KY; Kawata, H Structure-activity relationships of bioisosteric replacement of the carboxylic acid in novel androgen receptor pure antagonists. Bioorg Med Chem 18:3159-68 (2010) [PubMed] ArticleMore Info.:
Target
Name:
Androgen receptor
Synonyms:
ANDR_HUMAN | AR | Androgen Receptor | Androgen receptor (AR) | Androgen receptor/Baculoviral IAP repeat-containing protein 2 | DHTR | Dihydrotestosterone receptor | NR3C4 | Nuclear receptor subfamily 3 group C member 4
Type:
Receptor
Mol. Mass.:
99185.27
Organism:
Homo sapiens (Human)
Description:
CHO cells were stably transfected with human AR gene.
Residue:
920
Sequence:
MEVQLGLGRVYPRPPSKTYRGAFQNLFQSVREVIQNPGPRHPEAASAAPPGASLLLLQQQQQQQQQQQQQQQQQQQQQQQETSPRQQQQQQGEDGSPQAHRRGPTGYLVLDEEQQPSQPQSALECHPERGCVPEPGAAVAASKGLPQQLPAPPDEDDSAAPSTLSLLGPTFPGLSSCSADLKDILSEASTMQLLQQQQQEAVSEGSSSGRAREASGAPTSSKDNYLGGTSTISDNAKELCKAVSVSMGLGVEALEHLSPGEQLRGDCMYAPLLGVPPAVRPTPCAPLAECKGSLLDDSAGKSTEDTAEYSPFKGGYTKGLEGESLGCSGSAAAGSSGTLELPSTLSLYKSGALDEAAAYQSRDYYNFPLALAGPPPPPPPPHPHARIKLENPLDYGSAWAAAAAQCRYGDLASLHGAGAAGPGSGSPSAAASSSWHTLFTAEEGQLYGPCGGGGGGGGGGGGGGGGGGGGGGGEAGAVAPYGYTRPPQGLAGQESDFTAPDVWYPGGMVSRVPYPSPTCVKSEMGPWMDSYSGPYGDMRLETARDHVLPIDYYFPPQKTCLICGDEASGCHYGALTCGSCKVFFKRAAEGKQKYLCASRNDCTIDKFRRKNCPSCRLRKCYEAGMTLGARKLKKLGNLKLQEEGEASSTTSPTEETTQKLTVSHIEGYECQPIFLNVLEAIEPGVVCAGHDNNQPDSFAALLSSLNELGERQLVHVVKWAKALPGFRNLHVDDQMAVIQYSWMGLMVFAMGWRSFTNVNSRMLYFAPDLVFNEYRMHKSRMYSQCVRMRHLSQEFGWLQITPQEFLCMKALLLFSIIPVDGLKNQKFFDELRMNYIKELDRIIACKRKNPTSCSRRFYQLTKLLDSVQPIARELHQFTFDLLIKSHMVSVDFPEMMAEIISVQVPKILSGKVKPIYFHTQ
Inhibitor
Name:
BDBM50317665
Synonyms:
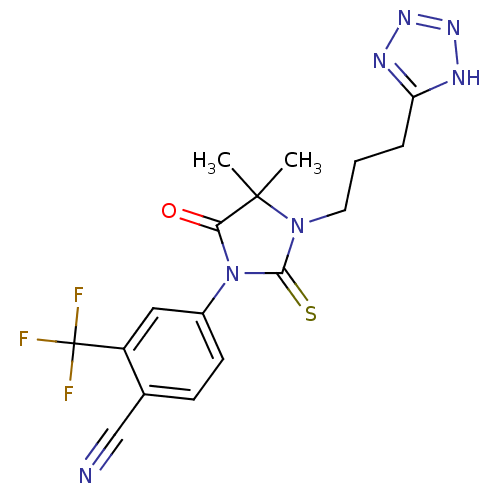
4-{4,4-Dimethyl-5-oxo-3-[3-(1H-tetrazol-5-yl)propyl]-2-thioxoimidazolidin-1-yl}-2-trifluoromethylbenzonitrile | CHEMBL1099158
Type:
Small organic molecule
Emp. Form.:
C17H16F3N7OS
Mol. Mass.:
423.415
SMILES:
CC1(C)N(CCCc2nnn[nH]2)C(=S)N(C1=O)c1ccc(C#N)c(c1)C(F)(F)F