Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Acetyl-CoA carboxylase 2
Ligand
BDBM50311816
Substrate
n/a
Meas. Tech.
ChEMBL_728690 (CHEMBL1685967)
IC50
23±n/a nM
Citation
 Chonan, T; Wakasugi, D; Yamamoto, D; Yashiro, M; Oi, T; Tanaka, H; Ohoka-Sugita, A; Io, F; Koretsune, H; Hiratate, A Discovery of novel (4-piperidinyl)-piperazines as potent and orally active acetyl-CoA carboxylase 1/2 non-selective inhibitors: F-Boc and triF-Boc groups are acid-stable bioisosteres for the Boc group. Bioorg Med Chem 19:1580-93 (2011) [PubMed] Article
Chonan, T; Wakasugi, D; Yamamoto, D; Yashiro, M; Oi, T; Tanaka, H; Ohoka-Sugita, A; Io, F; Koretsune, H; Hiratate, A Discovery of novel (4-piperidinyl)-piperazines as potent and orally active acetyl-CoA carboxylase 1/2 non-selective inhibitors: F-Boc and triF-Boc groups are acid-stable bioisosteres for the Boc group. Bioorg Med Chem 19:1580-93 (2011) [PubMed] ArticleMore Info.:
Target
Name:
Acetyl-CoA carboxylase 2
Synonyms:
ACACB | ACACB_HUMAN | ACC-beta | ACC2 | ACCB | Acetyl-CoA carboxylase | Acetyl-CoA carboxylase 2 | Acetyl-CoA carboxylase 2 (ACC) | Acetyl-CoA carboxylase 2 (ACC2)
Type:
Protein
Mol. Mass.:
276535.21
Organism:
Homo sapiens (Human)
Description:
O00763
Residue:
2458
Sequence:
MVLLLCLSCLIFSCLTFSWLKIWGKMTDSKPITKSKSEANLIPSQEPFPASDNSGETPQRNGEGHTLPKTPSQAEPASHKGPKDAGRRRNSLPPSHQKPPRNPLSSSDAAPSPELQANGTGTQGLEATDTNGLSSSARPQGQQAGSPSKEDKKQANIKRQLMTNFILGSFDDYSSDEDSVAGSSRESTRKGSRASLGALSLEAYLTTGEAETRVPTMRPSMSGLHLVKRGREHKKLDLHRDFTVASPAEFVTRFGGDRVIEKVLIANNGIAAVKCMRSIRRWAYEMFRNERAIRFVVMVTPEDLKANAEYIKMADHYVPVPGGPNNNNYANVELIVDIAKRIPVQAVWAGWGHASENPKLPELLCKNGVAFLGPPSEAMWALGDKIASTVVAQTLQVPTLPWSGSGLTVEWTEDDLQQGKRISVPEDVYDKGCVKDVDEGLEAAERIGFPLMIKASEGGGGKGIRKAESAEDFPILFRQVQSEIPGSPIFLMKLAQHARHLEVQILADQYGNAVSLFGRDCSIQRRHQKIVEEAPATIAPLAIFEFMEQCAIRLAKTVGYVSAGTVEYLYSQDGSFHFLELNPRLQVEHPCTEMIADVNLPAAQLQIAMGVPLHRLKDIRLLYGESPWGVTPISFETPSNPPLARGHVIAARITSENPDEGFKPSSGTVQELNFRSSKNVWGYFSVAATGGLHEFADSQFGHCFSWGENREEAISNMVVALKELSIRGDFRTTVEYLINLLETESFQNNDIDTGWLDYLIAEKVQAEKPDIMLGVVCGALNVADAMFRTCMTDFLHSLERGQVLPADSLLNLVDVELIYGGVKYILKVARQSLTMFVLIMNGCHIEIDAHRLNDGGLLLSYNGNSYTTYMKEEVDSYRITIGNKTCVFEKENDPTVLRSPSAGKLTQYTVEDGGHVEAGSSYAEMEVMKMIMTLNVQERGRVKYIKRPGAVLEAGCVVARLELDDPSKVHPAEPFTGELPAQQTLPILGEKLHQVFHSVLENLTNVMSGFCLPEPVFSIKLKEWVQKLMMTLRHPSLPLLELQEIMTSVAGRIPAPVEKSVRRVMAQYASNITSVLCQFPSQQIATILDCHAATLQRKADREVFFINTQSIVQLVQRYRSGIRGYMKTVVLDLLRRYLRVEHHFQQAHYDKCVINLREQFKPDMSQVLDCIFSHAQVAKKNQLVIMLIDELCGPDPSLSDELISILNELTQLSKSEHCKVALRARQILIASHLPSYELRHNQVESIFLSAIDMYGHQFCPENLKKLILSETTIFDVLPTFFYHANKVVCMASLEVYVRRGYIAYELNSLQHRQLPDGTCVVEFQFMLPSSHPNRMTVPISITNPDLLRHSTELFMDSGFSPLCQRMGAMVAFRRFEDFTRNFDEVISCFANVPKDTPLFSEARTSLYSEDDCKSLREEPIHILNVSIQCADHLEDEALVPILRTFVQSKKNILVDYGLRRITFLIAQEKEFPKFFTFRARDEFAEDRIYRHLEPALAFQLELNRMRNFDLTAVPCANHKMHLYLGAAKVKEGVEVTDHRFFIRAIIRHSDLITKEASFEYLQNEGERLLLEAMDELEVAFNNTSVRTDCNHIFLNFVPTVIMDPFKIEESVRYMVMRYGSRLWKLRVLQAEVKINIRQTTTGSAVPIRLFITNESGYYLDISLYKEVTDSRSGNIMFHSFGNKQGPQHGMLINTPYVTKDLLQAKRFQAQTLGTTYIYDFPEMFRQALFKLWGSPDKYPKDILTYTELVLDSQGQLVEMNRLPGGNEVGMVAFKMRFKTQEYPEGRDVIVIGNDITFRIGSFGPGEDLLYLRASEMARAEGIPKIYVAANSGARIGMAEEIKHMFHVAWVDPEDPHKGFKYLYLTPQDYTRISSLNSVHCKHIEEGGESRYMITDIIGKDDGLGVENLRGSGMIAGESSLAYEEIVTISLVTCRAIGIGAYLVRLGQRVIQVENSHIILTGASALNKVLGREVYTSNNQLGGVQIMHYNGVSHITVPDDFEGVYTILEWLSYMPKDNHSPVPIITPTDPIDREIEFLPSRAPYDPRWMLAGRPHPTLKGTWQSGFFDHGSFKEIMAPWAQTVVTGRARLGGIPVGVIAVETRTVEVAVPADPANLDSEAKIIQQAGQVWFPDSAYKTAQAVKDFNREKLPLMIFANWRGFSGGMKDMYDQVLKFGAYIVDGLRQYKQPILIYIPPYAELRGGSWVVIDATINPLCIEMYADKESRGGVLEPEGTVEIKFRKKDLIKSMRRIDPAYKKLMEQLGEPDLSDKDRKDLEGRLKAREDLLLPIYHQVAVQFADFHDTPGRMLEKGVISDILEWKTARTFLYWRLRRLLLEDQVKQEILQASGELSHVHIQSMLRRWFVETEGAVKAYLWDNNQVVVQWLEQHWQAGDGPRSTIRENITYLKHDSVLKTIRGLVEENPEVAVDCVIYLSQHISPAERAQVVHLLSTMDSPAST
Inhibitor
Name:
BDBM50311816
Synonyms:
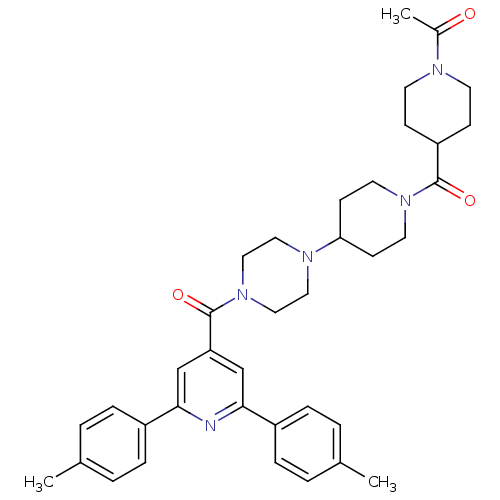
1-(4-(4-(4-(2,6-dip-tolylisonicotinoyl)piperazin-1-yl)piperidine-1-carbonyl)piperidin-1-yl)ethanone | CHEMBL1076223
Type:
Small organic molecule
Emp. Form.:
C37H45N5O3
Mol. Mass.:
607.7849
SMILES:
CC(=O)N1CCC(CC1)C(=O)N1CCC(CC1)N1CCN(CC1)C(=O)c1cc(nc(c1)-c1ccc(C)cc1)-c1ccc(C)cc1