Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50393734
Substrate
n/a
Meas. Tech.
ChEMBL_856844 (CHEMBL2163109)
IC50
>10000±n/a nM
Citation
 Bosnar, M; Kragol, G; Koštrun, S; Vujasinovic, I; Bošnjak, B; Bencetic Mihaljevic, V; Marušic Ištuk, Z; Kapic, S; Hrvacic, B; Brajša, K; Tavcar, B; Jelic, D; Glojnaric, I; Verbanac, D; Culic, O; Padovan, J; Alihodžic, S; Erakovic Haber, V; Spaventi, R N'-substituted-2'-O,3'-N-carbonimidoyl bridged macrolides: novel anti-inflammatory macrolides without antimicrobial activity. J Med Chem 55:6111-23 (2012) [PubMed] Article
Bosnar, M; Kragol, G; Koštrun, S; Vujasinovic, I; Bošnjak, B; Bencetic Mihaljevic, V; Marušic Ištuk, Z; Kapic, S; Hrvacic, B; Brajša, K; Tavcar, B; Jelic, D; Glojnaric, I; Verbanac, D; Culic, O; Padovan, J; Alihodžic, S; Erakovic Haber, V; Spaventi, R N'-substituted-2'-O,3'-N-carbonimidoyl bridged macrolides: novel anti-inflammatory macrolides without antimicrobial activity. J Med Chem 55:6111-23 (2012) [PubMed] ArticleMore Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
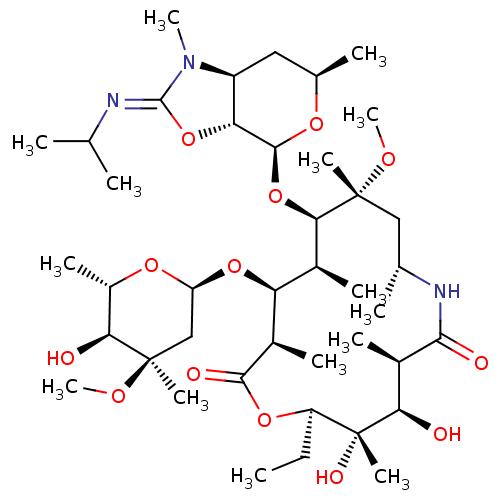
Inhibitor
Name:
BDBM50393734
Synonyms:
CHEMBL2159133
Type:
Small organic molecule
Emp. Form.:
C41H73N3O13
Mol. Mass.:
816.0306
SMILES:
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@H]3[C@H]2O\C(=N/C(C)C)N3C)[C@@](C)(C[C@@H](C)NC(=O)[C@H](C)[C@@H](O)[C@]1(C)O)OC |r|