Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
Ligand
BDBM50077360
Substrate
n/a
Meas. Tech.
ChEMBL_981761 (CHEMBL2426842)
IC50
35±n/a nM
Citation
 Yoshida, K; Itoyama, R; Yamahira, M; Tanaka, J; Loaëc, N; Lozach, O; Durieu, E; Fukuda, T; Ishibashi, F; Meijer, L; Iwao, M Synthesis, resolution, and biological evaluation of atropisomeric (aR)- and (aS)-16-methyllamellarins N: unique effects of the axial chirality on the selectivity of protein kinases inhibition. J Med Chem 56:7289-301 (2013) [PubMed] Article
Yoshida, K; Itoyama, R; Yamahira, M; Tanaka, J; Loaëc, N; Lozach, O; Durieu, E; Fukuda, T; Ishibashi, F; Meijer, L; Iwao, M Synthesis, resolution, and biological evaluation of atropisomeric (aR)- and (aS)-16-methyllamellarins N: unique effects of the axial chirality on the selectivity of protein kinases inhibition. J Med Chem 56:7289-301 (2013) [PubMed] ArticleMore Info.:
Target
Name:
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
Synonyms:
DYR1A_HUMAN | DYRK | DYRK1A | Dual specificity YAK1-related kinase | Dual specificity YAK1-related kinase 1A (Dyrk1A) | Dual specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A) | Dual-specificity tyrosine-phosphorylation regulated kinase 1A | Dual-specificity tyrosine-regulated kinases 1A | HP86 | MNB | MNBH
Type:
Enzyme
Mol. Mass.:
85616.61
Organism:
Homo sapiens (Human)
Description:
Q13627
Residue:
763
Sequence:
MHTGGETSACKPSSVRLAPSFSFHAAGLQMAGQMPHSHQYSDRRQPNISDQQVSALSYSDQIQQPLTNQVMPDIVMLQRRMPQTFRDPATAPLRKLSVDLIKTYKHINEVYYAKKKRRHQQGQGDDSSHKKERKVYNDGYDDDNYDYIVKNGEKWMDRYEIDSLIGKGSFGQVVKAYDRVEQEWVAIKIIKNKKAFLNQAQIEVRLLELMNKHDTEMKYYIVHLKRHFMFRNHLCLVFEMLSYNLYDLLRNTNFRGVSLNLTRKFAQQMCTALLFLATPELSIIHCDLKPENILLCNPKRSAIKIVDFGSSCQLGQRIYQYIQSRFYRSPEVLLGMPYDLAIDMWSLGCILVEMHTGEPLFSGANEVDQMNKIVEVLGIPPAHILDQAPKARKFFEKLPDGTWNLKKTKDGKREYKPPGTRKLHNILGVETGGPGGRRAGESGHTVADYLKFKDLILRMLDYDPKTRIQPYYALQHSFFKKTADEGTNTSNSVSTSPAMEQSQSSGTTSSTSSSSGGSSGTSNSGRARSDPTHQHRHSGGHFTAAVQAMDCETHSPQVRQQFPAPLGWSGTEAPTQVTVETHPVQETTFHVAPQQNALHHHHGNSSHHHHHHHHHHHHHGQQALGNRTRPRVYNSPTNSSSTQDSMEVGHSHHSMTSLSSSTTSSSTSSSSTGNQGNQAYQNRPVAANTLDFGQNGAMDVNLTVYSNPRQETGIAGHPTYQFSANTGPAHYMTEGHLTMRQGADREESPMTGVCVQQSPVASS
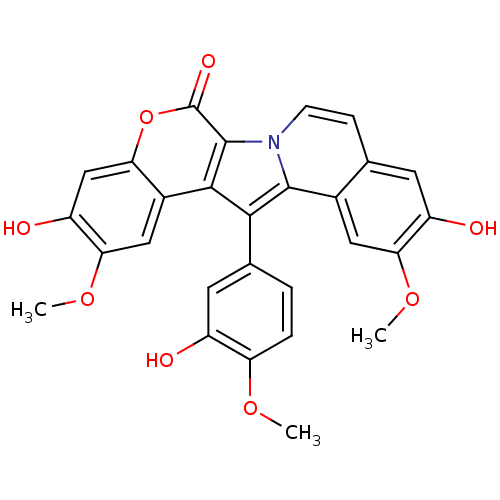
Inhibitor
Name:
BDBM50077360
Synonyms:
3,10-Dihydroxy-13-(3-hydroxy-4-methoxy-phenyl)-2,11-dimethoxy-5-oxa-6b-aza-dibenzo[a,i]fluoren-6-one | CHEMBL301226
Type:
Small organic molecule
Emp. Form.:
C28H21NO8
Mol. Mass.:
499.4682
SMILES:
COc1ccc(cc1O)-c1c2c(n3ccc4cc(O)c(OC)cc4c13)c(=O)oc1cc(O)c(OC)cc21