Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Phosphatidylinositol 3-kinase C2 domain-containing subunit gamma
Ligand
BDBM657053
Substrate
n/a
Meas. Tech.
PI3K-gamma Scintillation Proximity Assay
IC50
<100±n/a nM
Citation
 Shepard, S; Combs, AP; Falahatpisheh, N; Shao, L Aminopyrazine diol compounds as PI3K-γ inhibitors US Patent US11926616 Publication Date 3/12/2024
Shepard, S; Combs, AP; Falahatpisheh, N; Shao, L Aminopyrazine diol compounds as PI3K-γ inhibitors US Patent US11926616 Publication Date 3/12/2024More Info.:
Target
Name:
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
Synonyms:
PI3-kinase p110 subunit gamma | PI3-kinase subunit p120-gamma | PI3Kgamma | PIK3CG | PK3CG_HUMAN | Phosphatidylinositol 4,5-biphosphate 3-kinase catalytic subunit gamma (PIK3CG) | Phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase 110 kDa catalytic subunit gamma (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma (PI3Kgamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3Kgamma) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | Phosphoinositide 3-Kinase (PI3K), gamma Chain A | Phosphoinositide 3-kinases gamma (PI3K gamma) | Phosphoinositide-3-kinase (PI3K gamma) | p120-PI3K
Type:
Enzyme Subunit
Mol. Mass.:
126470.30
Organism:
Homo sapiens (Human)
Description:
P48736
Residue:
1102
Sequence:
MELENYKQPVVLREDNCRRRRRMKPRSAAASLSSMELIPIEFVLPTSQRKCKSPETALLHVAGHGNVEQMKAQVWLRALETSVAADFYHRLGPHHFLLLYQKKGQWYEIYDKYQVVQTLDCLRYWKATHRSPGQIHLVQRHPPSEESQAFQRQLTALIGYDVTDVSNVHDDELEFTRRGLVTPRMAEVASRDPKLYAMHPWVTSKPLPEYLWKKIANNCIFIVIHRSTTSQTIKVSPDDTPGAILQSFFTKMAKKKSLMDIPESQSEQDFVLRVCGRDEYLVGETPIKNFQWVRHCLKNGEEIHVVLDTPPDPALDEVRKEEWPLVDDCTGVTGYHEQLTIHGKDHESVFTVSLWDCDRKFRVKIRGIDIPVLPRNTDLTVFVEANIQHGQQVLCQRRTSPKPFTEEVLWNVWLEFSIKIKDLPKGALLNLQIYCGKAPALSSKASAESPSSESKGKVQLLYYVNLLLIDHRFLLRRGEYVLHMWQISGKGEDQGSFNADKLTSATNPDKENSMSISILLDNYCHPIALPKHQPTPDPEGDRVRAEMPNQLRKQLEAIIATDPLNPLTAEDKELLWHFRYESLKHPKAYPKLFSSVKWGQQEIVAKTYQLLARREVWDQSALDVGLTMQLLDCNFSDENVRAIAVQKLESLEDDDVLHYLLQLVQAVKFEPYHDSALARFLLKRGLRNKRIGHFLFWFLRSEIAQSRHYQQRFAVILEAYLRGCGTAMLHDFTQQVQVIEMLQKVTLDIKSLSAEKYDVSSQVISQLKQKLENLQNSQLPESFRVPYDPGLKAGALAIEKCKVMASKKKPLWLEFKCADPTALSNETIGIIFKHGDDLRQDMLILQILRIMESIWETESLDLCLLPYGCISTGDKIGMIEIVKDATTIAKIQQSTVGNTGAFKDEVLNHWLKEKSPTEEKFQAAVERFVYSCAGYCVATFVLGIGDRHNDNIMITETGNLFHIDFGHILGNYKSFLGINKERVPFVLTPDFLFVMGTSGKKTSPHFQKFQDICVKAYLALRHHTNLLIILFSMMLMTGMPQLTSKEDIEYIRDALTVGKNEEDAKKYFLDQIEVCRDKGWTVQFNWFLHLVLGIKQGEKHSA
Inhibitor
Name:
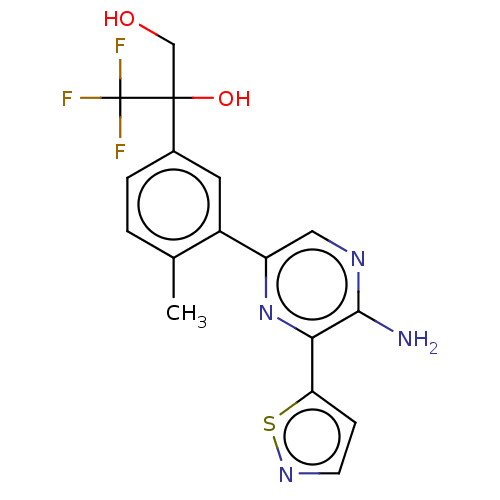
BDBM657053
Synonyms:
2-(3-(5-Amino-6-(isothiazol-5-yl)pyrazin-2- yl)-4-methylphenyl)-3,3,3-trifluoropropane- 1,2-diol, trifluoroacetate salt (single enantiomer isolated, believed to be (S)-2-(3- (5-Amino-6-(isothiazol-5-yl)pyrazin-2-yl)-4- methylphenyl)-3,3,3-trifluoropropane-1,2- diol, trifluoroacetate salt) | 2-(3-(5-Amino-6-(isothiazol-5-yl)pyrazin-2-yl)-4-methylphenyl)-3,3,3-trifluoropropane-1,2-diol | US11926616, Example 6
Type:
Small organic molecule
Emp. Form.:
C17H15F3N4O2S
Mol. Mass.:
396.387
SMILES:
Cc1ccc(cc1-c1cnc(N)c(n1)-c1ccns1)C(O)(CO)C(F)(F)F