Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 1A
Ligand
BDBM263383
Substrate
n/a
Meas. Tech.
5-HT1A Receptor Binding Test
pH
7.4±n/a
Ki
0.980±n/a nM
Comments
extracted
Citation
More Info.:
Target
Name:
5-hydroxytryptamine receptor 1A
Synonyms:
5-HT-1A | 5-HT1A | 5-hydroxytryptamine receptor 1A (5-HT-1A) | 5HT1A_HUMAN | ADRB2RL1 | ADRBRL1 | Dopamine D2 receptor and serotonin 1a receptor | G-21 | HTR1A | Serotonin receptor 1A
Type:
n/a
Mol. Mass.:
46122.49
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
422
Sequence:
MDVLSPGQGNNTTSPPAPFETGGNTTGISDVTVSYQVITSLLLGTLIFCAVLGNACVVAAIALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCCTSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPEDRSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVKKVEKTGADTRHGASPAPQPKKSVNGESGSRNWRLGVESKAGGALCANGAVRQGDDGAALEVIEVHRVGNSKEHLPLPSEAGPTPCAPASFERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLPFFIVALVLPFCESSCHMPTLLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFCRQ
Inhibitor
Name:
BDBM263383
Synonyms:
US9550741, I-17
Type:
Small organic molecule
Emp. Form.:
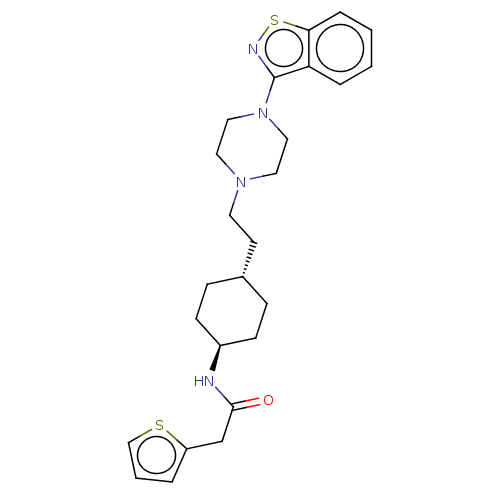
C25H32N4OS2
Mol. Mass.:
468.678
SMILES:
O=C(Cc1cccs1)N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1 |r,wU:9.9,wD:12.13,(-6.35,1.05,;-6.35,2.59,;-7.69,3.36,;-9.02,2.59,;-9.5,4.05,;-11.04,4.05,;-11.51,2.59,;-10.27,1.68,;-5.02,3.36,;-3.69,2.59,;-2.35,3.36,;-1.02,2.59,;-1.02,1.05,;.32,.28,;1.65,1.05,;2.98,.28,;2.98,-1.26,;4.32,-2.03,;5.65,-1.26,;5.65,.28,;4.32,1.05,;6.98,-2.03,;6.98,-3.57,;8.45,-4.05,;9.35,-2.8,;10.89,-2.64,;11.51,-1.24,;10.61,.01,;9.08,-.15,;8.45,-1.56,;-2.35,.28,;-3.69,1.05,)|