Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Gastrin/cholecystokinin type B receptor
Ligand
BDBM50472875
Substrate
n/a
Meas. Tech.
ChEMBL_48108 (CHEMBL663095)
Ki
1.3±n/a nM
Citation
 Ursini, A; Capelli, AM; Carr, RA; CassarÓ, P; Corsi, M; Curcuruto, O; Curotto, G; Dal Cin, M; Davalli, S; Donati, D; Feriani, A; Finch, H; Finizia, G; Gaviraghi, G; Marien, M; Pentassuglia, G; Polinelli, S; Ratti, E; Reggiani, AM; Tarzia, G; Tedesco, G; Tranquillini, ME; Trist, DG; Van Amsterdam, FT; Reggiani, A Synthesis and SAR of new 5-phenyl-3-ureido-1,5-benzodiazepines as cholecystokinin-B receptor antagonists. J Med Chem 43:3596-613 (2000) [PubMed] Article
Ursini, A; Capelli, AM; Carr, RA; CassarÓ, P; Corsi, M; Curcuruto, O; Curotto, G; Dal Cin, M; Davalli, S; Donati, D; Feriani, A; Finch, H; Finizia, G; Gaviraghi, G; Marien, M; Pentassuglia, G; Polinelli, S; Ratti, E; Reggiani, AM; Tarzia, G; Tedesco, G; Tranquillini, ME; Trist, DG; Van Amsterdam, FT; Reggiani, A Synthesis and SAR of new 5-phenyl-3-ureido-1,5-benzodiazepines as cholecystokinin-B receptor antagonists. J Med Chem 43:3596-613 (2000) [PubMed] ArticleMore Info.:
Target
Name:
Gastrin/cholecystokinin type B receptor
Synonyms:
CCK-2 receptor | CCK-B receptor | CCK-BR | CCKBR | CCKRB | Cholecystokinin A | Cholecystokinin receptor | Cholecystokinin-2 Receptor | GASR_HUMAN | Gastrin/cholecystokinin type B receptor
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
48445.79
Organism:
Homo sapiens (Human)
Description:
Stable expression of human CCK-2 receptors in HEK 293 cells.
Residue:
447
Sequence:
MELLKLNRSVQGTGPGPGASLCRPGAPLLNSSSVGNLSCEPPRIRGAGTRELELAIRITLYAVIFLMSVGGNMLIIVVLGLSRRLRTVTNAFLLSLAVSDLLLAVACMPFTLLPNLMGTFIFGTVICKAVSYLMGVSVSVSTLSLVAIALERYSAICRPLQARVWQTRSHAARVIVATWLLSGLLMVPYPVYTVVQPVGPRVLQCVHRWPSARVRQTWSVLLLLLLFFIPGVVMAVAYGLISRELYLGLRFDGDSDSDSQSRVRNQGGLPGAVHQNGRCRPETGAVGEDSDGCYVQLPRSRPALELTALTAPGPGSGSRPTQAKLLAKKRVVRMLLVIVVLFFLCWLPVYSANTWRAFDGPGAHRALSGAPISFIHLLSYASACVNPLVYCFMHRRFRQACLETCARCCPRPPRARPRALPDEDPPTPSIASLSRLSYTTISTLGPG
Inhibitor
Name:
BDBM50472875
Synonyms:
CHEMBL333930
Type:
Small organic molecule
Emp. Form.:
C35H39N5O3
Mol. Mass.:
577.7159
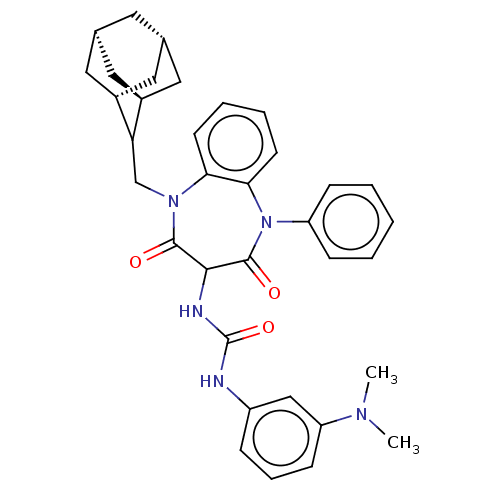
SMILES:
[H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(CN1c4ccccc4N(c4ccccc4)C(=O)C(NC(=O)Nc4cccc(c4)N(C)C)C1=O)[C@@]([H])(C2)C3 |wU:3.3,43.48,wD:1.0,6.6,TLB:10:9:5:1.8.2,THB:10:9:1.8.45:3.46.5,45:43:5:1.8.2,45:1:9.43.46:5,2:3:9:1.8.45,2:1:9:3.46.5,(12.5,-.13,;11.22,-.95,;10.02,-.03,;8.72,-.77,;7.54,.18,;8.29,-2.13,;9.48,-3.02,;8.17,-3.79,;10.65,-2.27,;9.75,-4.71,;8.71,-5.83,;9.15,-7.29,;8.18,-8.23,;6.84,-7.45,;5.51,-8.23,;5.51,-9.77,;6.84,-10.55,;8.18,-9.79,;9.12,-10.69,;8.65,-12.15,;9.7,-13.29,;9.25,-14.75,;7.73,-15.09,;6.68,-13.96,;7.16,-12.49,;10.61,-10.43,;11.53,-11.69,;11.34,-9.02,;12.88,-9.06,;13.63,-10.4,;12.84,-11.72,;15.17,-10.42,;15.92,-11.77,;15.14,-13.07,;15.87,-14.43,;17.42,-14.46,;18.21,-13.12,;17.45,-11.77,;19.73,-13.13,;20.47,-14.47,;20.5,-11.83,;10.65,-7.56,;11.6,-6.35,;10.33,-3.28,;11.48,-4.27,;11.61,-2.52,;9.07,-2.33,)|