Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Fibroblast growth factor receptor 2
Ligand
BDBM249396
Substrate
n/a
Meas. Tech.
ChEMBL_2123936 (CHEMBL4833169)
IC50
1290±n/a nM
Citation
More Info.:
Target
Name:
Fibroblast growth factor receptor 2
Synonyms:
BEK | CD_antigen=CD332 | FGFR-2 | FGFR-2 Tyrosine Kinase | FGFR2 | FGFR2_HUMAN | Fibroblast growth factor receptor 2 (FGFR2) | Fibroblast growth factor receptor 2 precursor | KGFR | KSAM | Keratinocyte growth factor receptor | Keratinocyte growth factor receptor 2 | VEGF-receptor 2 and Fibroblast growth factor receptor 2
Type:
Enzyme
Mol. Mass.:
92015.45
Organism:
Homo sapiens (Human)
Description:
P21802
Residue:
821
Sequence:
MVSWGRFICLVVVTMATLSLARPSFSLVEDTTLEPEEPPTKYQISQPEVYVAAPGESLEVRCLLKDAAVISWTKDGVHLGPNNRTVLIGEYLQIKGATPRDSGLYACTASRTVDSETWYFMVNVTDAISSGDDEDDTDGAEDFVSENSNNKRAPYWTNTEKMEKRLHAVPAANTVKFRCPAGGNPMPTMRWLKNGKEFKQEHRIGGYKVRNQHWSLIMESVVPSDKGNYTCVVENEYGSINHTYHLDVVERSPHRPILQAGLPANASTVVGGDVEFVCKVYSDAQPHIQWIKHVEKNGSKYGPDGLPYLKVLKAAGVNTTDKEIEVLYIRNVTFEDAGEYTCLAGNSIGISFHSAWLTVLPAPGREKEITASPDYLEIAIYCIGVFLIACMVVTVILCRMKNTTKKPDFSSQPAVHKLTKRIPLRRQVTVSAESSSSMNSNTPLVRITTRLSSTADTPMLAGVSEYELPEDPKWEFPRDKLTLGKPLGEGCFGQVVMAEAVGIDKDKPKEAVTVAVKMLKDDATEKDLSDLVSEMEMMKMIGKHKNIINLLGACTQDGPLYVIVEYASKGNLREYLRARRPPGMEYSYDINRVPEEQMTFKDLVSCTYQLARGMEYLASQKCIHRDLAARNVLVTENNVMKIADFGLARDINNIDYYKKTTNGRLPVKWMAPEALFDRVYTHQSDVWSFGVLMWEIFTLGGSPYPGIPVEELFKLLKEGHRMDKPANCTNELYMMMRDCWHAVPSQRPTFKQLVEDLDRILTLTTNEEYLDLSQPLEQYSPSYPDTRSSCSSGDDSVFSPDPMPYEPCLPQYPHINGSVKT
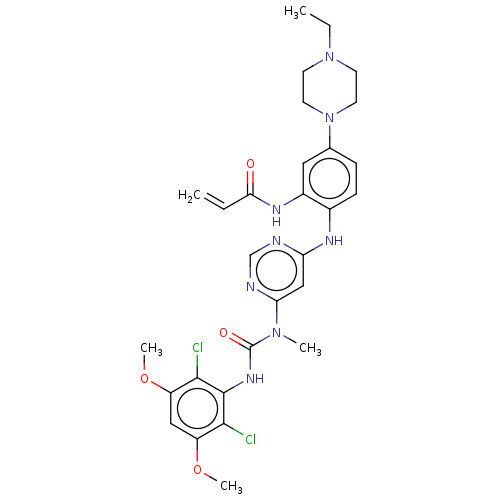
Inhibitor
Name:
BDBM249396
Synonyms:
US10537571, Compound 108 | US10562888, Compound 108 | US10654836, The compound of example 108 of WO2015057938 | US10912774, Compound 108 | US11001572, Example 108 of WO2015057938 | US9434697, 108 | US9730931, Compound 108
Type:
Small organic molecule
Emp. Form.:
C29H34Cl2N8O4
Mol. Mass.:
629.537
SMILES:
CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)c(NC(=O)C=C)c1