Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Adenosine receptor A2a
Ligand
BDBM50177309
Substrate
n/a
Meas. Tech.
ChEMBL_334364 (CHEMBL858945)
Ki
>10000±n/a nM
Citation
More Info.:
Target
Name:
Adenosine receptor A2a
Synonyms:
A2A adenosine receptor (hA2A) | AA2AR_HUMAN | ADENOSINE A2 | ADENOSINE A2a | ADORA2 | ADORA2A | Adenosine A2A receptor (A2AAR)
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
44716.46
Organism:
Homo sapiens (Human)
Description:
P29274
Residue:
412
Sequence:
MPIMGSSVYITVELAIAVLAILGNVLVCWAVWLNSNLQNVTNYFVVSLAAADIAVGVLAIPFAITISTGFCAACHGCLFIACFVLVLTQSSIFSLLAIAIDRYIAIRIPLRYNGLVTGTRAKGIIAICWVLSFAIGLTPMLGWNNCGQPKEGKNHSQGCGEGQVACLFEDVVPMNYMVYFNFFACVLVPLLLMLGVYLRIFLAARRQLKQMESQPLPGERARSTLQKEVHAAKSLAIIVGLFALCWLPLHIINCFTFFCPDCSHAPLWLMYLAIVLSHTNSVVNPFIYAYRIREFRQTFRKIIRSHVLRQQEPFKAAGTSARVLAAHGSDGEQVSLRLNGHPPGVWANGSAPHPERRPNGYALGLVSGGSAQESQGNTGLPDVELLSHELKGVCPEPPGLDDPLAQDGAGVS
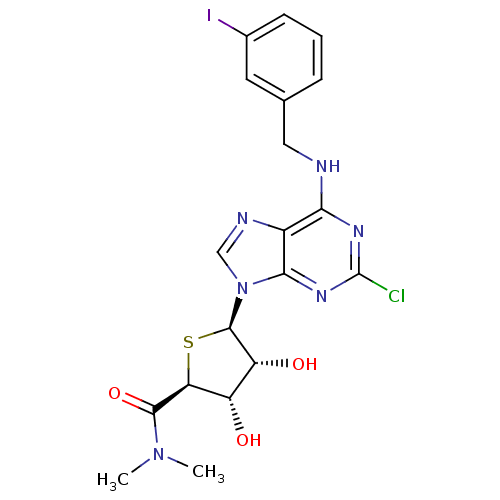
Inhibitor
Name:
BDBM50177309
Synonyms:
(2S,3S,4R,5R)-5-(6-(3-iodobenzylamino)-2-chloro-9H-purin-9-yl)-3,4-dihydroxy-N,N-dimethyl-tetrahydrothiophene-2-carboxamide | (2S,3S,4R,5R)-5-[2-chloro-6-(3-iodo-benzylamino)-purin-9-yl]-3,4-dihydroxy-tetrahydro-thiophene-2-carboxylic aciddimethylamide | CHEMBL436485
Type:
Small organic molecule
Emp. Form.:
C19H20ClIN6O3S
Mol. Mass.:
574.823
SMILES:
CN(C)C(=O)[C@H]1S[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r|