Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM295665
Substrate
n/a
Meas. Tech.
ChEMBL_2240001 (CHEMBL5153897)
IC50
>10000±n/a nM
Citation
 Hanan, EJ; Braun, MG; Heald, RA; MacLeod, C; Chan, C; Clausen, S; Edgar, KA; Eigenbrot, C; Elliott, R; Endres, N; Friedman, LS; Gogol, E; Gu, XH; Thibodeau, RH; Jackson, PS; Kiefer, JR; Knight, JD; Nannini, M; Narukulla, R; Pace, A; Pang, J; Purkey, HE; Salphati, L; Sampath, D; Schmidt, S; Sideris, S; Song, K; Sujatha-Bhaskar, S; Ultsch, M; Wallweber, H; Xin, J; Yeap, S; Young, A; Zhong, Y; Staben, ST Discovery of GDC-0077 (Inavolisib), a Highly Selective Inhibitor and Degrader of Mutant PI3K?. J Med Chem 65:16589-16621 (2022) [PubMed] Article
Hanan, EJ; Braun, MG; Heald, RA; MacLeod, C; Chan, C; Clausen, S; Edgar, KA; Eigenbrot, C; Elliott, R; Endres, N; Friedman, LS; Gogol, E; Gu, XH; Thibodeau, RH; Jackson, PS; Kiefer, JR; Knight, JD; Nannini, M; Narukulla, R; Pace, A; Pang, J; Purkey, HE; Salphati, L; Sampath, D; Schmidt, S; Sideris, S; Song, K; Sujatha-Bhaskar, S; Ultsch, M; Wallweber, H; Xin, J; Yeap, S; Young, A; Zhong, Y; Staben, ST Discovery of GDC-0077 (Inavolisib), a Highly Selective Inhibitor and Degrader of Mutant PI3K?. J Med Chem 65:16589-16621 (2022) [PubMed] ArticleMore Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
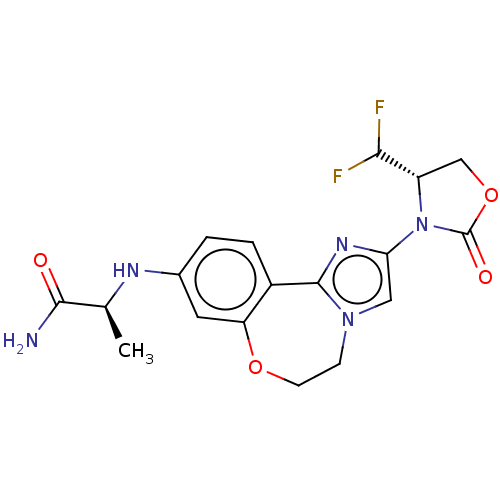
BDBM295665
Synonyms:
(S)-2-((2-((S)-4-(difluoromethyl)- 2-oxooxazolidin-3-yl)-5,6- dihydrobenzo[f]imidazo[1,2- d][1,4]oxazepin-9- yl)amino)propanamide | US10112932, Compound 101 | US10851091, Compound 101
Type:
Small organic molecule
Emp. Form.:
C18H19F2N5O4
Mol. Mass.:
407.3714
SMILES:
C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C(N)=O |r|