Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Maltase-glucoamylase
Ligand
BDBM50242271
Substrate
n/a
Meas. Tech.
ChEMBL_2265471
IC50
154300±n/a nM
Citation
 Wang, KM; Ge, YX; Zhang, J; Chen, YT; Zhang, NY; Gu, JS; Fang, L; Zhang, XL; Zhang, J; Jiang, CS New cycloalkyl[b]thiophenylnicotinamide-based ?-glucosidase inhibitors as promising anti-diabetic agents: Synthesis, in silico study, in vitro and in vivo evaluations. Bioorg Med Chem Lett 79:0 (2023) [PubMed]
Wang, KM; Ge, YX; Zhang, J; Chen, YT; Zhang, NY; Gu, JS; Fang, L; Zhang, XL; Zhang, J; Jiang, CS New cycloalkyl[b]thiophenylnicotinamide-based ?-glucosidase inhibitors as promising anti-diabetic agents: Synthesis, in silico study, in vitro and in vivo evaluations. Bioorg Med Chem Lett 79:0 (2023) [PubMed]More Info.:
Target
Name:
Maltase-glucoamylase
Synonyms:
Alpha glucosidase | Alpha-1,4-glucosidase | Glucan 1,4-alpha-glucosidase | MGA | MGAM | MGAML | MGA_HUMAN | Maltase | Maltase-glucoamylase, intestinal | Synonyms=MGA
Type:
Enzyme
Mol. Mass.:
209817.06
Organism:
Homo sapiens (Human)
Description:
O43451
Residue:
2753
Sequence:
MARKKLKKFTTLEIVLSVLLLVLFIISIVLIVLLAKESLKSTAPDPGTTGTPDPGTTGTPDPGTTGTTHARTTGPPDPGTTGTTPVSAECPVVNELERINCIPDQPPTKATCDQRGCCWNPQGAVSVPWCYYSKNHSYHVEGNLVNTNAGFTARLKNLPSSPVFGSNVDNVLLTAEYQTSNRFHFKLTDQTNNRFEVPHEHVQSFSGNAAASLTYQVEISRQPFSIKVTRRSNNRVLFDSSIGPLLFADQFLQLSTRLPSTNVYGLGEHVHQQYRHDMNWKTWPIFNRDTTPNGNGTNLYGAQTFFLCLEDASGLSFGVFLMNSNAMEVVLQPAPAITYRTIGGILDFYVFLGNTPEQVVQEYLELIGRPALPSYWALGFHLSRYEYGTLDNMREVVERNRAAQLPYDVQHADIDYMDERRDFTYDSVDFKGFPEFVNELHNNGQKLVIIVDPAISNNSSSSKPYGPYDRGSDMKIWVNSSDGVTPLIGEVWPGQTVFPDYTNPNCAVWWTKEFELFHNQVEFDGIWIDMNEVSNFVDGSVSGCSTNNLNNPPFTPRILDGYLFCKTLCMDAVQHWGKQYDIHNLYGYSMAVATAEAAKTVFPNKRSFILTRSTFAGSGKFAAHWLGDNTATWDDLRWSIPGVLEFNLFGIPMVGPDICGFALDTPEELCRRWMQLGAFYPFSRNHNGQGYKDQDPASFGADSLLLNSSRHYLNIRYTLLPYLYTLFFRAHSRGDTVARPLLHEFYEDNSTWDVHQQFLWGPGLLITPVLDEGAEKVMAYVPDAVWYDYETGSQVRWRKQKVEMELPGDKIGLHLRGGYIFPTQQPNTTTLASRKNPLGLIIALDENKEAKGELFWDNGETKDTVANKVYLLCEFSVTQNRLEVNISQSTYKDPNNLAFNEIKILGTEEPSNVTVKHNGVPSQTSPTVTYDSNLKVAIITDIDLLLGEAYTVEWSIKIRDEEKIDCYPDENGASAENCTARGCIWEASNSSGVPFCYFVNDLYSVSDVQYNSHGATADISLKSSVYANAFPSTPVNPLRLDVTYHKNEMLQFKIYDPNKNRYEVPVPLNIPSMPSSTPEGQLYDVLIKKNPFGIEIRRKSTGTIIWDSQLLGFTFSDMFIRISTRLPSKYLYGFGETEHRSYRRDLEWHTWGMFSRDQPPGYKKNSYGVHPYYMGLEEDGSAHGVLLLNSNAMDVTFQPLPALTYRTTGGVLDFYVFLGPTPELVTQQYTELIGRPVMVPYWSLGFQLCRYGYQNDSEIASLYDEMVAAQIPYDVQYSDIDYMERQLDFTLSPKFAGFPALINRMKADGMRVILILDPAISGNETQPYPAFTRGVEDDVFIKYPNDGDIVWGKVWPDFPDVVVNGSLDWDSQVELYRAYVAFPDFFRNSTAKWWKREIEELYNNPQNPERSLKFDGMWIDMNEPSSFVNGAVSPGCRDASLNHPPYMPHLESRDRGLSSKTLCMESQQILPDGSLVQHYNVHNLYGWSQTRPTYEAVQEVTGQRGVVITRSTFPSSGRWAGHWLGDNTAAWDQLKKSIIGMMEFSLFGISYTGADICGFFQDAEYEMCVRWMQLGAFYPFSRNHNTIGTRRQDPVSWDAAFVNISRNVLQTRYTLLPYLYTLMQKAHTEGVTVVRPLLHEFVSDQVTWDIDSQFLLGPAFLVSPVLERNARNVTAYFPRARWYDYYTGVDINARGEWKTLPAPLDHINLHVRGGYILPWQEPALNTHLSRKNPLGLIIALDENKEAKGELFWDDGQTKDTVAKKVYLLCEFSVTQNHLEVTISQSTYKDPNNLAFNEIKILGMEEPSNVTVKHNGVPSQTSPTVTYDSNLKVAIITDINLFLGEAYTVEWSIKIRDEEKIDCYPDENGDSAENCTARGCIWEASNSSGVPFCYFVNDLYSVSDVQYNSHGATADISLKSSVHANAFPSTPVNPLRLDVTYHKNEMLQFKIYDPNNNRYEVPVPLNIPSVPSSTPEGQLYDVLIKKNPFGIEIRRKSTGTIIWDSQLLGFTFNDMFIRISTRLPSKYLYGFGETEHTSYRRDLEWHTWGMFSRDQPPGYKKNSYGVHPYYMGLEEDGSAHGVLLLNSNAMDVTFQPLPALTYRTTGGVLDFYVFLGPTPELVTQQYTELIGRPVMVPYWSLGFQLCRYGYQNDSEISSLYDEMVAAQIPYDVQYSDIDYMERQLDFTLSPKFAGFPALINRMKADGMRVILILDPAISGNETQPYPAFTRGVEDDVFIKYPNDGDIVWGKVWPDFPDVVVNGSLDWDSQVELYRAYVAFPDFFRNSTAKWWKREIEELYNNPQNPERSLKFDGMWIDMNEPSSFVNGAVSPGCRDASLNHPPYMPYLESRDRGLSSKTLCMESQQILPDGSPVQHYNVHNLYGWSQTRPTYEAVQEVTGQRGVVITRSTFPSSGRWAGHWLGDNTAAWDQLKKSIIGMMEFSLFGISYTGADICGFFQDAEYEMCVRWMQLGAFYPFSRNHNTIGTRRQDPVSWDVAFVNISRTVLQTRYTLLPYLYTLMHKAHTEGVTVVRPLLHEFVSDQVTWDIDSQFLLGPAFLVSPVLERNARNVTAYFPRARWYDYYTGVDINARGEWKTLPAPLDHINLHVRGGYILPWQEPALNTHLSRQKFMGFKIALDDEGTAGGWLFWDDGQSIDTYGKGLYYLASFSASQNTMQSHIIFNNYITGTNPLKLGYIEIWGVGSVPVTSVSISVSGMVITPSFNNDPTTQVLSIDVTDRNISLHNFTSLTWISTL
Inhibitor
Name:
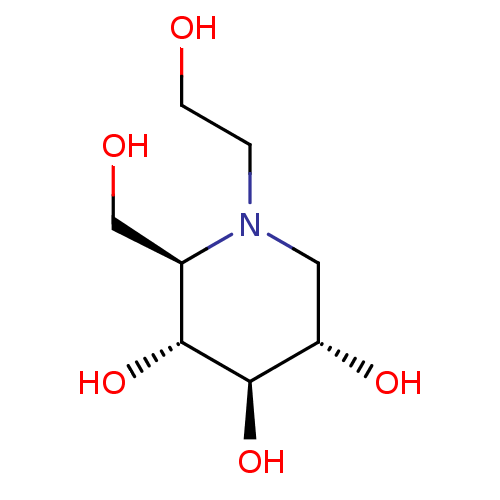
BDBM50242271
Synonyms:
(2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)piperidine-3,4,5-triol | (2R,3R,4R,5S)-1-ethoxy-2-(hydroxymethyl)piperidine-3,4,5-triol | BAY-M-1099 | CHEMBL1561 | Glyset | MIGLITOL | N-Hydroxyethyl-1-deoxynojirimycin | cid_441314
Type:
Small organic molecule
Emp. Form.:
C8H17NO5
Mol. Mass.:
207.2243
SMILES:
OCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r|