Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
RAC-alpha serine/threonine-protein kinase
Ligand
BDBM50326198
Substrate
n/a
Meas. Tech.
ChEMBL_659348 (CHEMBL1247975)
IC50
>8000±n/a nM
Citation
 Cee, VJ; Schenkel, LB; Hodous, BL; Deak, HL; Nguyen, HN; Olivieri, PR; Romero, K; Bak, A; Be, X; Bellon, S; Bush, TL; Cheng, AC; Chung, G; Coats, S; Eden, PM; Hanestad, K; Gallant, PL; Gu, Y; Huang, X; Kendall, RL; Lin, MH; Morrison, MJ; Patel, VF; Radinsky, R; Rose, PE; Ross, S; Sun, JR; Tang, J; Zhao, H; Payton, M; Geuns-Meyer, SD Discovery of a potent, selective, and orally bioavailable pyridinyl-pyrimidine phthalazine aurora kinase inhibitor. J Med Chem 53:6368-77 (2010) [PubMed] Article
Cee, VJ; Schenkel, LB; Hodous, BL; Deak, HL; Nguyen, HN; Olivieri, PR; Romero, K; Bak, A; Be, X; Bellon, S; Bush, TL; Cheng, AC; Chung, G; Coats, S; Eden, PM; Hanestad, K; Gallant, PL; Gu, Y; Huang, X; Kendall, RL; Lin, MH; Morrison, MJ; Patel, VF; Radinsky, R; Rose, PE; Ross, S; Sun, JR; Tang, J; Zhao, H; Payton, M; Geuns-Meyer, SD Discovery of a potent, selective, and orally bioavailable pyridinyl-pyrimidine phthalazine aurora kinase inhibitor. J Med Chem 53:6368-77 (2010) [PubMed] ArticleMore Info.:
Target
Name:
RAC-alpha serine/threonine-protein kinase
Synonyms:
AKT phosphorylation (p-AKT) | AKT1 | AKT1/PPP1CA | AKT1_HUMAN | C-AKT | PKB | PKB alpha | Protein kinase Akt-1 | Protein kinase B | Protein kinase B (AKT1) | Protein kinase B (Akt 1) | Protein kinase B (Akt) | Protein kinase B alpha | Protein kinase B alpha (AKT1) | Proto-oncogene Akt (Akt1) | Proto-oncogene c-Akt (AKT) | Proto-oncogene c-Akt (AKT1) | RAC | RAC-PK-alpha | RAC-alpha serine/threonine-protein kinase (AKT) | RAC-alpha serine/threonine-protein kinase (AKT1) | RAC-alpha serine/threonine-protein kinase (pAKT)
Type:
Enzyme
Mol. Mass.:
55681.25
Organism:
Homo sapiens (Human)
Description:
P31749
Residue:
480
Sequence:
MSDVAIVKEGWLHKRGEYIKTWRPRYFLLKNDGTFIGYKERPQDVDQREAPLNNFSVAQCQLMKTERPRPNTFIIRCLQWTTVIERTFHVETPEEREEWTTAIQTVADGLKKQEEEEMDFRSGSPSDNSGAEEMEVSLAKPKHRVTMNEFEYLKLLGKGTFGKVILVKEKATGRYYAMKILKKEVIVAKDEVAHTLTENRVLQNSRHPFLTALKYSFQTHDRLCFVMEYANGGELFFHLSRERVFSEDRARFYGAEIVSALDYLHSEKNVVYRDLKLENLMLDKDGHIKITDFGLCKEGIKDGATMKTFCGTPEYLAPEVLEDNDYGRAVDWWGLGVVMYEMMCGRLPFYNQDHEKLFELILMEEIRFPRTLGPEAKSLLSGLLKKDPKQRLGGGSEDAKEIMQHRFFAGIVWQHVYEKKLSPPFKPQVTSETDTRYFDEEFTAQMITITPPDQDDSMECVDSERRPHFPQFSYSASGTA
Inhibitor
Name:
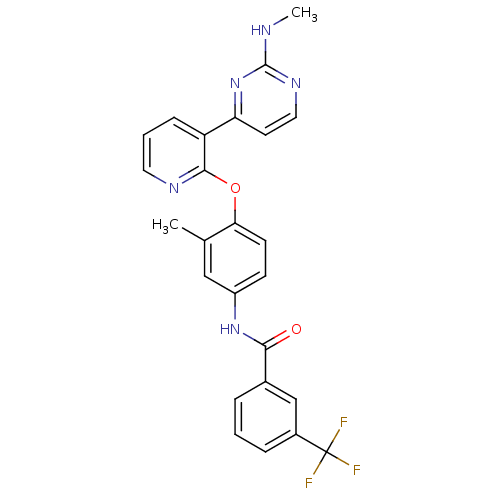
BDBM50326198
Synonyms:
CHEMBL1242977 | N-(3-Methyl-4-(3-(2-(methylamino)pyrimidin-4-yl)pyridin-2-yloxy)phenyl)-3-(trifluoromethyl)benzamide
Type:
Small organic molecule
Emp. Form.:
C25H20F3N5O2
Mol. Mass.:
479.4538
SMILES:
CNc1nccc(n1)-c1cccnc1Oc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1C