Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Ubiquitin carboxyl-terminal hydrolase 7
Ligand
BDBM50241487
Substrate
n/a
Meas. Tech.
ChEMBL_2274024
IC50
>50000±n/a nM
Citation
More Info.:
Target
Name:
Ubiquitin carboxyl-terminal hydrolase 7
Synonyms:
Deubiquitinating enzyme 7 | HAUSP | Herpesvirus-associated ubiquitin-specific protease | UBP7_HUMAN | USP7 | Ubiquitin thioesterase 7 | Ubiquitin-specific-processing protease 7
Type:
PROTEIN
Mol. Mass.:
128274.45
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1469483
Residue:
1102
Sequence:
MNHQQQQQQQKAGEQQLSEPEDMEMEAGDTDDPPRITQNPVINGNVALSDGHNTAEEDMEDDTSWRSEATFQFTVERFSRLSESVLSPPCFVRNLPWKIMVMPRFYPDRPHQKSVGFFLQCNAESDSTSWSCHAQAVLKIINYRDDEKSFSRRISHLFFHKENDWGFSNFMAWSEVTDPEKGFIDDDKVTFEVFVQADAPHGVAWDSKKHTGYVGLKNQGATCYMNSLLQTLFFTNQLRKAVYMMPTEGDDSSKSVPLALQRVFYELQHSDKPVGTKKLTKSFGWETLDSFMQHDVQELCRVLLDNVENKMKGTCVEGTIPKLFRGKMVSYIQCKEVDYRSDRREDYYDIQLSIKGKKNIFESFVDYVAVEQLDGDNKYDAGEHGLQEAEKGVKFLTLPPVLHLQLMRFMYDPQTDQNIKINDRFEFPEQLPLDEFLQKTDPKDPANYILHAVLVHSGDNHGGHYVVYLNPKGDGKWCKFDDDVVSRCTKEEAIEHNYGGHDDDLSVRHCTNAYMLVYIRESKLSEVLQAVTDHDIPQQLVERLQEEKRIEAQKRKERQEAHLYMQVQIVAEDQFCGHQGNDMYDEEKVKYTVFKVLKNSSLAEFVQSLSQTMGFPQDQIRLWPMQARSNGTKRPAMLDNEADGNKTMIELSDNENPWTIFLETVDPELAASGATLPKFDKDHDVMLFLKMYDPKTRSLNYCGHIYTPISCKIRDLLPVMCDRAGFIQDTSLILYEEVKPNLTERIQDYDVSLDKALDELMDGDIIVFQKDDPENDNSELPTAKEYFRDLYHRVDVIFCDKTIPNDPGFVVTLSNRMNYFQVAKTVAQRLNTDPMLLQFFKSQGYRDGPGNPLRHNYEGTLRDLLQFFKPRQPKKLYYQQLKMKITDFENRRSFKCIWLNSQFREEEITLYPDKHGCVRDLLEECKKAVELGEKASGKLRLLEIVSYKIIGVHQEDELLECLSPATSRTFRIEEIPLDQVDIDKENEMLVTVAHFHKEVFGTFGIPFLLRIHQGEHFREVMKRIQSLLDIQEKEFEKFKFAIVMMGRHQYINEDEYEVNLKDFEPQPGNMSHPRPWLGLDHFNKAPKRSRYTYLEKAIKIHN
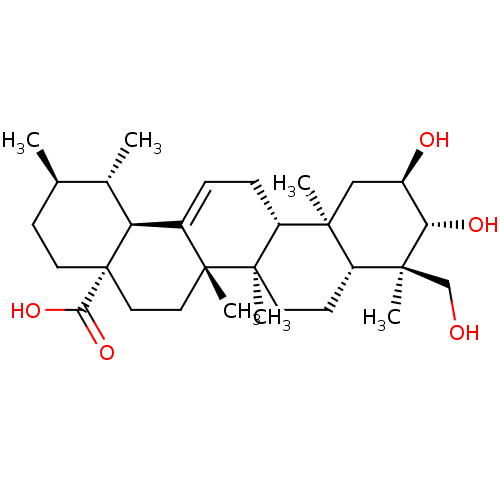
Inhibitor
Name:
BDBM50241487
Synonyms:
2,3,6,23-tetrahydroxyurs-12-en-28-oic acid | Asiatic acid | CHEMBL404313 | asiantic acid
Type:
Small organic molecule
Emp. Form.:
C30H48O5
Mol. Mass.:
488.6991
SMILES:
C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)C[C@@H](O)[C@H](O)[C@@](C)(CO)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9|