Reaction Details  Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Transcription initiation factor TFIID subunit 1
Ligand
BDBM321437
Substrate
n/a
Meas. Tech.
ChEMBL_2301526
Kd
>10000±n/a nM
Citation
 Crawford, TD; Vartanian, S; Côté, A; Bellon, S; Duplessis, M; Flynn, EM; Hewitt, M; Huang, HR; Kiefer, JR; Murray, J; Nasveschuk, CG; Pardo, E; Romero, FA; Sandy, P; Tang, Y; Taylor, AM; Tsui, V; Wang, J; Wang, S; Zawadzke, L; Albrecht, BK; Magnuson, SR; Cochran, AG; Stokoe, D Inhibition of bromodomain-containing protein 9 for the prevention of epigenetically-defined drug resistance. Bioorg Med Chem Lett 27:3534-3541 (2017) [PubMed]
Crawford, TD; Vartanian, S; Côté, A; Bellon, S; Duplessis, M; Flynn, EM; Hewitt, M; Huang, HR; Kiefer, JR; Murray, J; Nasveschuk, CG; Pardo, E; Romero, FA; Sandy, P; Tang, Y; Taylor, AM; Tsui, V; Wang, J; Wang, S; Zawadzke, L; Albrecht, BK; Magnuson, SR; Cochran, AG; Stokoe, D Inhibition of bromodomain-containing protein 9 for the prevention of epigenetically-defined drug resistance. Bioorg Med Chem Lett 27:3534-3541 (2017) [PubMed]More Info.:
Target
Name:
Transcription initiation factor TFIID subunit 1
Synonyms:
BA2R | BA2R | CCG1 | CCGS | Cell cycle gene 1 protein | TAF(II)250 | TAF1 | TAF1_HUMAN | TAF2A | TAFII-250 | TAFII250 | TBP-associated factor 250 kDa | Transcription initiation factor TFIID 250 kDa subunit | Transcription initiation factor TFIID subunit 1 | p250
Type:
PROTEIN
Mol. Mass.:
212598.75
Organism:
Homo sapiens (Human)
Description:
ChEMBL_108248
Residue:
1872
Sequence:
MGPGCDLLLRTAATITAAAIMSDTDSDEDSAGGGPFSLAGFLFGNINGAGQLEGESVLDDECKKHLAGLGALGLGSLITELTANEELTGTDGALVNDEGWVRSTEDAVDYSDINEVAEDESRRYQQTMGSLQPLCHSDYDEDDYDADCEDIDCKLMPPPPPPPGPMKKDKDQDSITGEKVDFSSSSDSESEMGPQEATQAESEDGKLTLPLAGIMQHDATKLLPSVTELFPEFRPGKVLRFLRLFGPGKNVPSVWRSARRKRKKKHRELIQEEQIQEVECSVESEVSQKSLWNYDYAPPPPPEQCLSDDEITMMAPVESKFSQSTGDIDKVTDTKPRVAEWRYGPARLWYDMLGVPEDGSGFDYGFKLRKTEHEPVIKSRMIEEFRKLEENNGTDLLADENFLMVTQLHWEDDIIWDGEDVKHKGTKPQRASLAGWLPSSMTRNAMAYNVQQGFAATLDDDKPWYSIFPIDNEDLVYGRWEDNIIWDAQAMPRLLEPPVLTLDPNDENLILEIPDEKEEATSNSPSKESKKESSLKKSRILLGKTGVIKEEPQQNMSQPEVKDPWNLSNDEYYYPKQQGLRGTFGGNIIQHSIPAVELRQPFFPTHMGPIKLRQFHRPPLKKYSFGALSQPGPHSVQPLLKHIKKKAKMREQERQASGGGEMFFMRTPQDLTGKDGDLILAEYSEENGPLMMQVGMATKIKNYYKRKPGKDPGAPDCKYGETVYCHTSPFLGSLHPGQLLQAFENNLFRAPIYLHKMPETDFLIIRTRQGYYIRELVDIFVVGQQCPLFEVPGPNSKRANTHIRDFLQVFIYRLFWKSKDRPRRIRMEDIKKAFPSHSESSIRKRLKLCADFKRTGMDSNWWVLKSDFRLPTEEEIRAMVSPEQCCAYYSMIAAEQRLKDAGYGEKSFFAPEEENEEDFQMKIDDEVRTAPWNTTRAFIAAMKGKCLLEVTGVADPTGCGEGFSYVKIPNKPTQQKDDKEPQPVKKTVTGTDADLRRLSLKNAKQLLRKFGVPEEEIKKLSRWEVIDVVRTMSTEQARSGEGPMSKFARGSRFSVAEHQERYKEECQRIFDLQNKVLSSTEVLSTDTDSSSAEDSDFEEMGKNIENMLQNKKTSSQLSREREEQERKELQRMLLAAGSAASGNNHRDDDTASVTSLNSSATGRCLKIYRTFRDEEGKEYVRCETVRKPAVIDAYVRIRTTKDEEFIRKFALFDEQHREEMRKERRRIQEQLRRLKRNQEKEKLKGPPEKKPKKMKERPDLKLKCGACGAIGHMRTNKFCPLYYQTNAPPSNPVAMTEEQEEELEKTVIHNDNEELIKVEGTKIVLGKQLIESADEVRRKSLVLKFPKQQLPPKKKRRVGTTVHCDYLNRPHKSIHRRRTDPMVTLSSILESIINDMRDLPNTYPFHTPVNAKVVKDYYKIITRPMDLQTLRENVRKRLYPSREEFREHLELIVKNSATYNGPKHSLTQISQSMLDLCDEKLKEKEDKLARLEKAINPLLDDDDQVAFSFILDNIVTQKMMAVPDSWPFHHPVNKKFVPDYYKVIVNPMDLETIRKNISKHKYQSRESFLDDVNLILANSVKYNGPESQYTKTAQEIVNVCYQTLTEYDEHLTQLEKDICTAKEAALEEAELESLDPMTPGPYTPQPPDLYDTNTSLSMSRDASVFQDESNMSVLDIPSATPEKQVTQEGEDGDGDLADEEEGTVQQPQASVLYEDLLMSEGEDDEEDAGSDEEGDNPFSAIQLSESGSDSDVGSGGIRPKQPRMLQENTRMDMENEESMMSYEGDGGEASHGLEDSNISYGSYEEPDPKSNTQDTSFSSIGGYEVSEEEEDEEEEEQRSGPSVLSQVHLSEDEEDSEDFHSIAGDSDLDSDE
Inhibitor
Name:
BDBM321437
Synonyms:
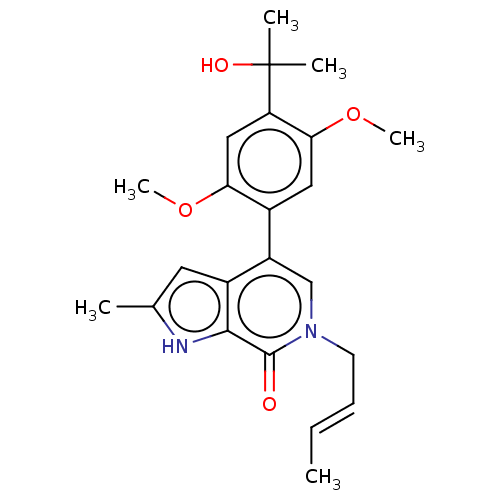
(E)-6-(but-2-en-1-yl)-4-(4-(2-hydroxypropan-2-yl)-2,5-dimethoxyphenyl)-2-methyl-1H-pyrrolo[2,3-c]pyridin-7(6H)-one | US10183009, Example 119
Type:
Small organic molecule
Emp. Form.:
C23H28N2O4
Mol. Mass.:
396.4794
SMILES:
COc1cc(c(OC)cc1-c1cn(C\C=C\C)c(=O)c2[nH]c(C)cc12)C(C)(C)O