

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A7 (Homo sapiens) | BDBM50318484 (2-((1R,6R)-6-Isopropenyl-3-methyl-cyclohex-2-enyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of human recombinant CYP3A7 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 30 mins f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00724 BindingDB Entry DOI: 10.7270/Q2697778 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 (Homo sapiens (Human)) | BDBM50508661 (CHEMBL4524830) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human CYP450 | Bioorg Med Chem Lett 29: 659-663 (2019) Article DOI: 10.1016/j.bmcl.2018.12.013 BindingDB Entry DOI: 10.7270/Q2K64NC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A7 (Homo sapiens) | BDBM50584760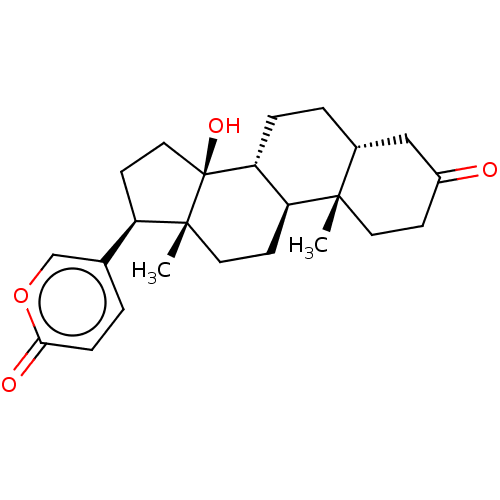 (CHEMBL2068968) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of recombinant human CYP3A7 incubated for 30 mins in presence of NADPH generating system by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01875 BindingDB Entry DOI: 10.7270/Q2PZ5DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A43/3A5/3A7 (Homo sapiens (Human)) | BDBM50464556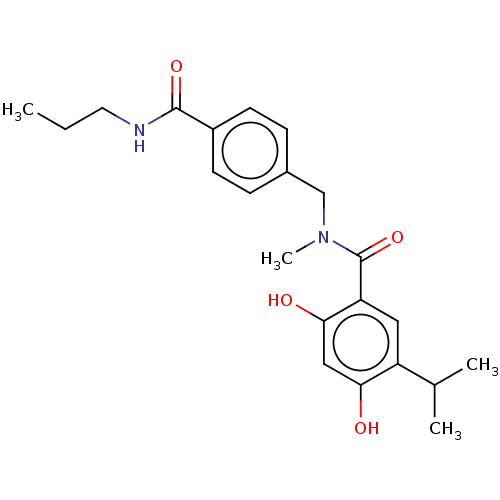 (CHEMBL4289811 | US10464907, Compound 21f | US10889...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Keimyung University Curated by ChEMBL | Assay Description Inhibition of CYP3A in human pooled human liver microsomes using midazolam as substrate assessed as midazolam 1'-hydroxylation preincubated for 5 min... | Eur J Med Chem 143: 390-401 (2018) Article DOI: 10.1016/j.ejmech.2017.11.054 BindingDB Entry DOI: 10.7270/Q29C713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A7 [T409R] (Homo sapiens (Human)) | BDBM50088503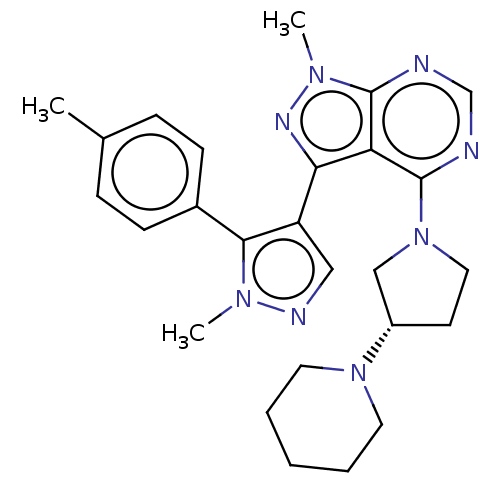 (CHEMBL3527048) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Reversible inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A7 harboring human P450 oxidoreductase and b5 | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A43/3A5/3A7 (Homo sapiens (Human)) | BDBM50508419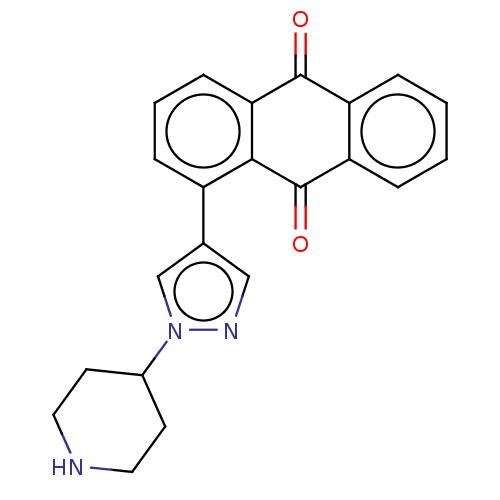 (CHEMBL4471264) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gwangju Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of CYP3A in human liver microsomes assessed as reduction in midazolam 1'-hydroxylation by tandem mass spectrometry analysis | J Med Chem 62: 575-588 (2019) Article DOI: 10.1021/acs.jmedchem.8b01168 BindingDB Entry DOI: 10.7270/Q2QZ2F8H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A43/3A5/3A7 (Homo sapiens (Human)) | BDBM50527104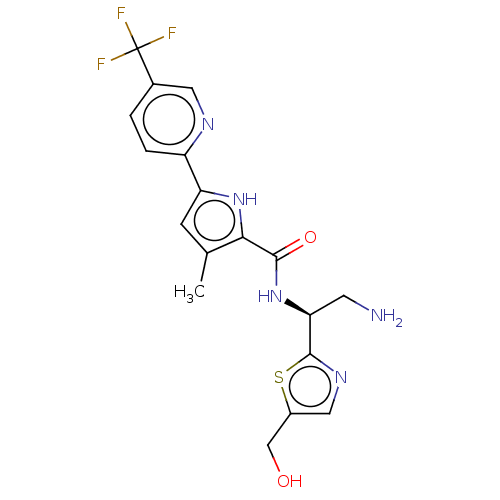 (CHEMBL4447583) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >6.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsley F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A in human liver microsomes using testosterone as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 ... | J Med Chem 63: 1724-1749 (2020) Article DOI: 10.1021/acs.jmedchem.9b02149 BindingDB Entry DOI: 10.7270/Q2X63RC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A7 (Homo sapiens) | BDBM50584760 (CHEMBL2068968) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of recombinant human CYP3A7 using midazolam as substrate preincubated for 30 mins in presence of NADPH generating system fo... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01875 BindingDB Entry DOI: 10.7270/Q2PZ5DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A7 (Homo sapiens) | BDBM50584761 (BUFALIN | Bufalin | CHEBI:517248) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of recombinant human CYP3A7 incubated for 30 mins in presence of NADPH generating system by LC-MS/MS analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01875 BindingDB Entry DOI: 10.7270/Q2PZ5DQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4/3A43/3A5/3A7 (Homo sapiens (Human)) | BDBM50527104 (CHEMBL4447583) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lindsley F. Kimball Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP3A in human liver microsomes using midazolam as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 to ... | J Med Chem 63: 1724-1749 (2020) Article DOI: 10.1021/acs.jmedchem.9b02149 BindingDB Entry DOI: 10.7270/Q2X63RC7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||