

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM12884 (5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-methoxy-phenyl)sulfonyl]amino]propyl]-2-oxo-3-phenyloxazolidine-5-carboxamide::(5S)-N-[(2S,3R)-3-hydroxy-4-[(4-methoxybenzene)(2-methylpropyl)sulfonamido]-1-phenylbutan-2-yl]-2-oxo-3-phenyl-1,3-oxazolidine-5-carboxamide::N-Aryl-oxazolidinone-5-carboxamide Analogue 21a
SMILES: COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CN(C(=O)O1)c1ccccc1
InChI Key: InChIKey=IGYIMSLYOKOAJR-NHKHRBQYSA-N
Data: 4 KI
PDB links: 2 PDB IDs contain this monomer as substructures. 2 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 Protease (Human immunodeficiency virus type 1) | BDBM12884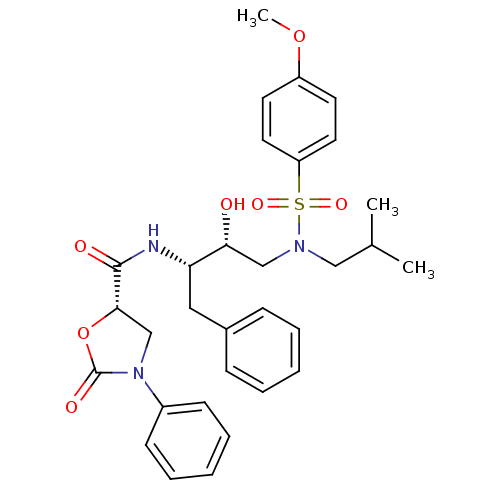 ((5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-...) | PDB MMDB B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School | Assay Description HIV-1 protease inhibitor activities were determined by the fluorescence resonance energy transfer (FRET) method. The energy transfer donor (EDANS) an... | J Med Chem 49: 7342-56 (2006) Article DOI: 10.1021/jm060666p BindingDB Entry DOI: 10.7270/Q25T3HQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM12884 ((5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12884 ((5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12884 ((5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||