

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM4685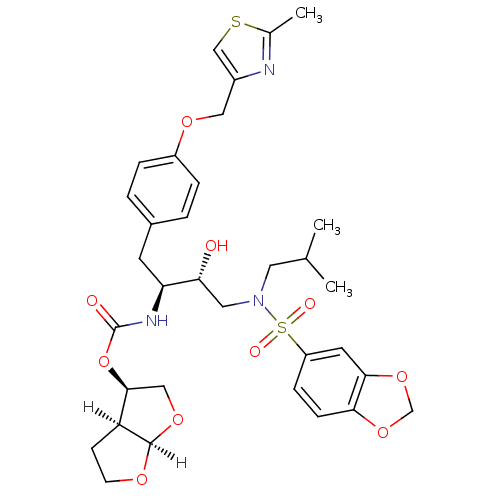 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0000150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Antimicrob Agents Chemother 51: 3147-54 (2007) Article DOI: 10.1128/aac.00401-07 BindingDB Entry DOI: 10.7270/Q23R0WPV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0000570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HIV1 protease by fluorescent peptide substrate based assay | Antimicrob Agents Chemother 51: 3147-54 (2007) Article DOI: 10.1128/aac.00401-07 BindingDB Entry DOI: 10.7270/Q23R0WPV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.000200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET assay | J Med Chem 55: 6328-41 (2012) Article DOI: 10.1021/jm300238h BindingDB Entry DOI: 10.7270/Q28G8PKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12877 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM12877 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12877 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease L10F/L19I/K20R/L33F/E35D/M36I/R41K/F53L/I54V/L63P/H69K/A71V/T74P/I84V/L89M/L90M/I93L mutant expressed in Esch... | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485746 (CHEMBL2165886) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET assay | J Med Chem 55: 6328-41 (2012) Article DOI: 10.1021/jm300238h BindingDB Entry DOI: 10.7270/Q28G8PKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM12877 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET assay | J Med Chem 55: 6328-41 (2012) Article DOI: 10.1021/jm300238h BindingDB Entry DOI: 10.7270/Q28G8PKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease I50V, A71V mutant by FRET assay | J Med Chem 55: 6328-41 (2012) Article DOI: 10.1021/jm300238h BindingDB Entry DOI: 10.7270/Q28G8PKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50505279 (CHEMBL4436207) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using RE(Edans)SGIFLETSK(Dabcyl)R as substrate by fluorescence method | J Med Chem 62: 9375-9414 (2019) Article DOI: 10.1021/acs.jmedchem.9b00359 BindingDB Entry DOI: 10.7270/Q2BR8WFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50469417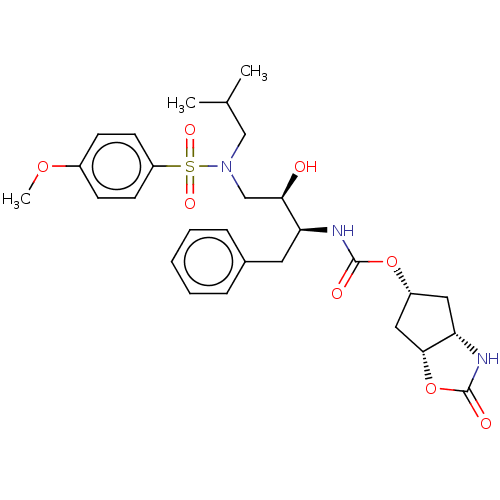 (CHEMBL4293023) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 wild type NL4-3 protease expressed in Escherichia coli Rosetta (DE3) pLysS using Abz-Thr-Ile-Nle-Phe-(pNO2)-Gln-Arg-NH2 as substra... | J Med Chem 61: 9722-9737 (2018) Article DOI: 10.1021/acs.jmedchem.8b01227 BindingDB Entry DOI: 10.7270/Q25T3P6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Antimicrob Agents Chemother 51: 3147-54 (2007) Article DOI: 10.1128/aac.00401-07 BindingDB Entry DOI: 10.7270/Q23R0WPV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498523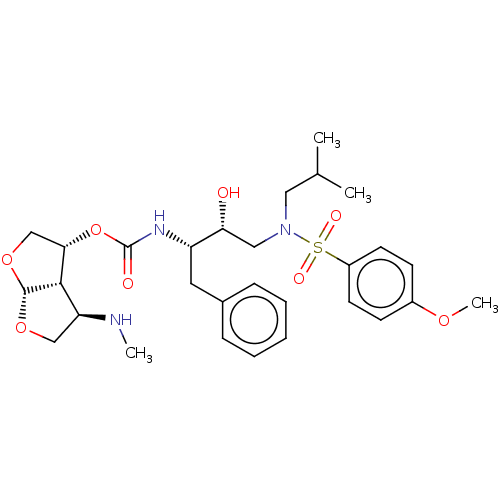 (CHEMBL3605643) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484842 (CHEMBL1958482 | GRL-0249A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528152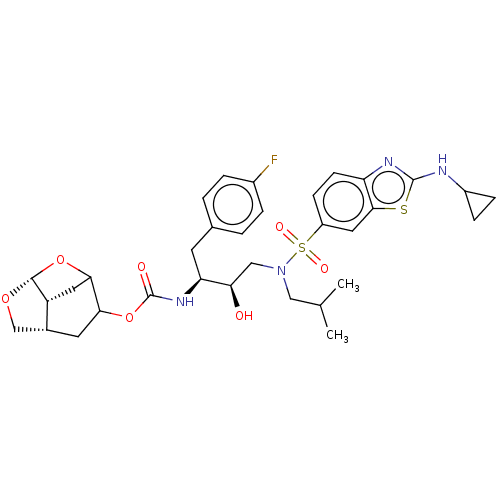 (CHEMBL4532946) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50528147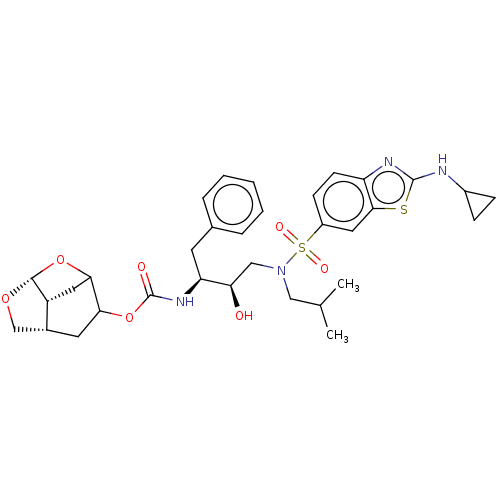 (CHEMBL4514504) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease | J Med Chem 63: 4867-4879 (2020) Article DOI: 10.1021/acs.jmedchem.0c00202 BindingDB Entry DOI: 10.7270/Q20V8H82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457611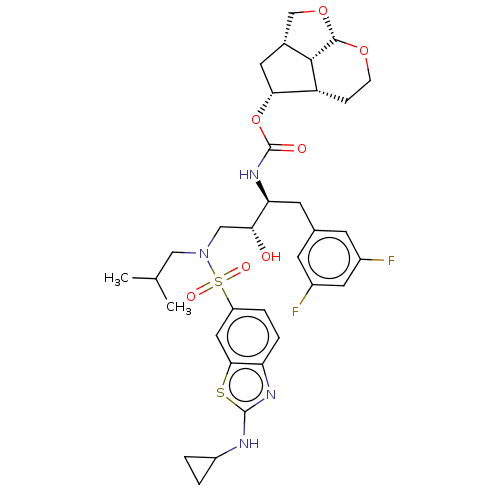 (CHEMBL4214453) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930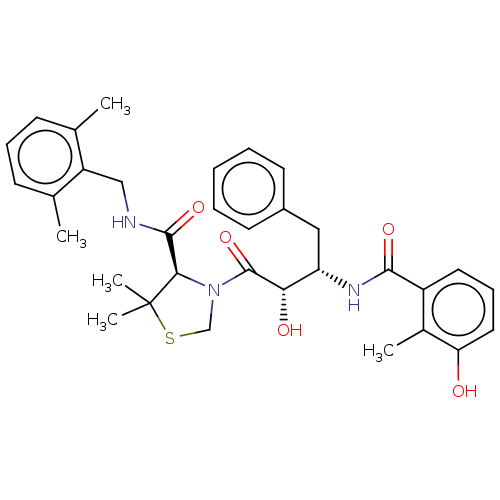 (CHEMBL584130 | KNI-814) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay | J Med Chem 52: 7604-17 (2009) Article DOI: 10.1021/jm9005115 BindingDB Entry DOI: 10.7270/Q2FR00F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483336 (CHEMBL1651153 | GRL-0476) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 (HIV-1)) | BDBM128418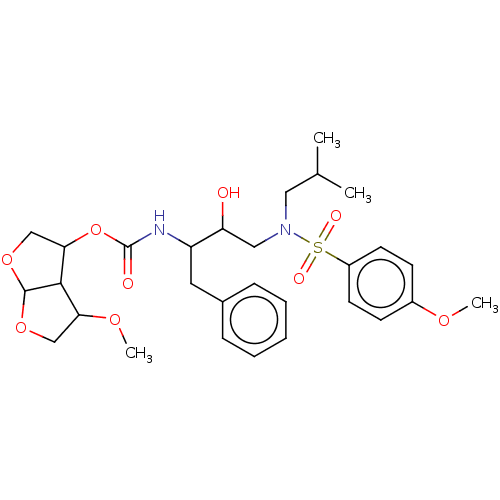 (US8802724, 23a | US8802724, 23c) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | 0.00290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Research Foundation US Patent | Assay Description The enzyme inhibitory activity (Ki) was determined according to an assay protocol reported by Toth and Marshall (Toth, M. V.; Marshall, G. R. Int. J.... | US Patent US8802724 (2014) BindingDB Entry DOI: 10.7270/Q2445K5D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485744 (CHEMBL2165917) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET assay | J Med Chem 55: 6328-41 (2012) Article DOI: 10.1021/jm300238h BindingDB Entry DOI: 10.7270/Q28G8PKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483092 (CHEMBL1276087) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498230 (CHEMBL3577576) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 58: 5088-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00474 BindingDB Entry DOI: 10.7270/Q27D2Z42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485740 (CHEMBL2165903) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET assay | J Med Chem 55: 6328-41 (2012) Article DOI: 10.1021/jm300238h BindingDB Entry DOI: 10.7270/Q28G8PKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485764 (CHEMBL2165912) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET assay | J Med Chem 55: 6328-41 (2012) Article DOI: 10.1021/jm300238h BindingDB Entry DOI: 10.7270/Q28G8PKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13925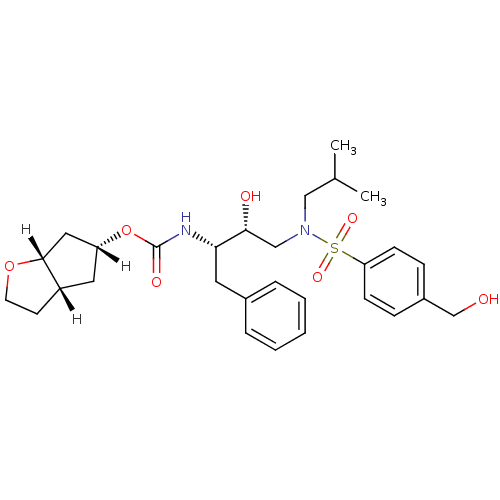 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University Graduate School of Medical and Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization in MT2 cells | J Biol Chem 282: 28709-20 (2007) Article DOI: 10.1074/jbc.M703938200 BindingDB Entry DOI: 10.7270/Q2JS9T6J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498524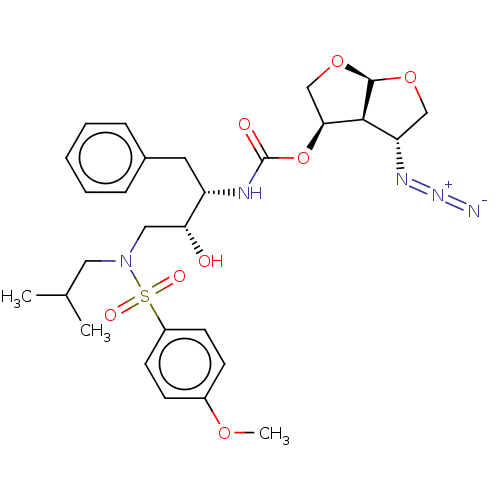 (CHEMBL3605638) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 58: 6994-7006 (2015) Article DOI: 10.1021/acs.jmedchem.5b00900 BindingDB Entry DOI: 10.7270/Q2862KFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12878 ((5S)-3-(4-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484847 (CHEMBL1958483 | GRL-0289A) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using hexapeptide Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fluorimetric assay | Bioorg Med Chem Lett 22: 2308-11 (2012) Article DOI: 10.1016/j.bmcl.2012.01.061 BindingDB Entry DOI: 10.7270/Q2B85C0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 protease L10I, G48V, I54V, L63P and V82A mutant by FRET assay | J Med Chem 55: 6328-41 (2012) Article DOI: 10.1021/jm300238h BindingDB Entry DOI: 10.7270/Q28G8PKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12878 ((5S)-3-(4-Acetylphenyl)-N-[(1S,2R)-1-benzyl-2-hydr...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease A71V/V82T/I84V mutant expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50457604 (CHEMBL4213229) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of wild type HIV-1 NL4-3 protease expressed in Escherichia coli Rosetta (DE3)pLysS using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 as substrate by fl... | J Med Chem 61: 4561-4577 (2018) Article DOI: 10.1021/acs.jmedchem.8b00298 BindingDB Entry DOI: 10.7270/Q2445Q35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM112661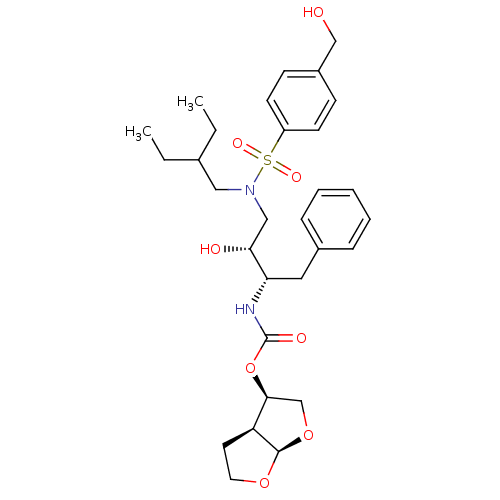 ((3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl ((2S,3...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of HIV1 NL4-3 protease I84V mutant expressed in Escherichia coli TAP-106 cells using EDANS/DABCYL-labelled 10-amino acid containing natura... | J Med Chem 62: 8062-8079 (2019) Article DOI: 10.1021/acs.jmedchem.9b00838 BindingDB Entry DOI: 10.7270/Q26H4MQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia State University Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease | J Med Chem 58: 5088-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b00474 BindingDB Entry DOI: 10.7270/Q27D2Z42 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484190 (CHEMBL1817686) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50180655 (A-157378-0 | A-157378.0 | ABT-378 | CHEBI:31781 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge Curated by ChEMBL | Assay Description Binding affinity to wild type HIV1 protease | J Med Chem 55: 1424-44 (2012) Article DOI: 10.1021/jm2010332 BindingDB Entry DOI: 10.7270/Q2D79F84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485760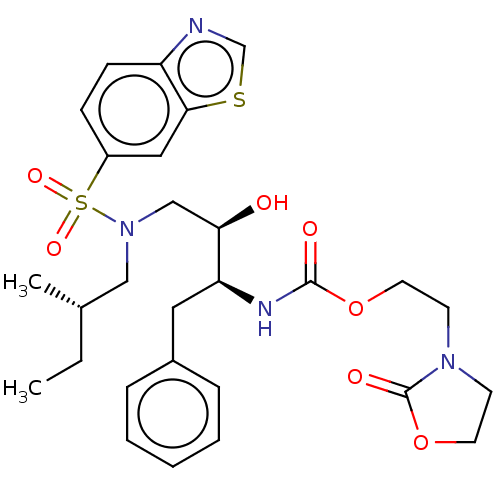 (CHEMBL2165901) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET assay | J Med Chem 55: 6328-41 (2012) Article DOI: 10.1021/jm300238h BindingDB Entry DOI: 10.7270/Q28G8PKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485747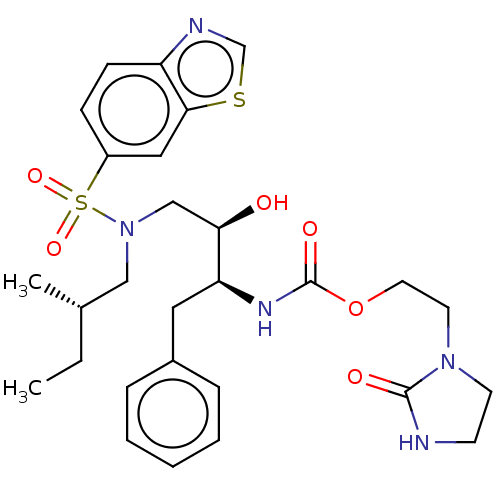 (CHEMBL2165900) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET assay | J Med Chem 55: 6328-41 (2012) Article DOI: 10.1021/jm300238h BindingDB Entry DOI: 10.7270/Q28G8PKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483338 (CHEMBL1651155) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 54: 622-34 (2011) Article DOI: 10.1021/jm1012787 BindingDB Entry DOI: 10.7270/Q2BV7KG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13925 ((3aS,5R,6aR)-hexahydro-2H-cyclopenta[b]furan-5-yl ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem 15: 7576-80 (2007) Article DOI: 10.1016/j.bmc.2007.09.010 BindingDB Entry DOI: 10.7270/Q2VD726P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50481584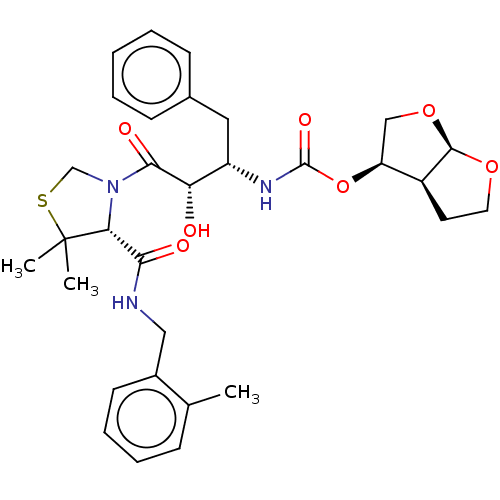 (CHEMBL589988 | GRL-0355) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Bioorg Med Chem Lett 20: 1241-6 (2010) Article DOI: 10.1016/j.bmcl.2009.11.123 BindingDB Entry DOI: 10.7270/Q2CJ8HB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.00530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assay | J Med Chem 51: 4839-43 (2008) Article DOI: 10.1021/jm8002334 BindingDB Entry DOI: 10.7270/Q24B344W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12883 ((5S)-3-(3-Acetylphenyl)-N-[(1S,2R)-3-[[(benzo[1,3]...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50489369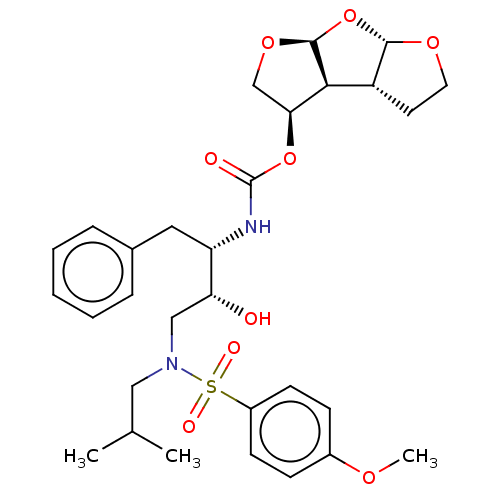 (CHEMBL1232930 | GRL-0519) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 56: 6792-802 (2013) Article DOI: 10.1021/jm400768f BindingDB Entry DOI: 10.7270/Q2K07764 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483116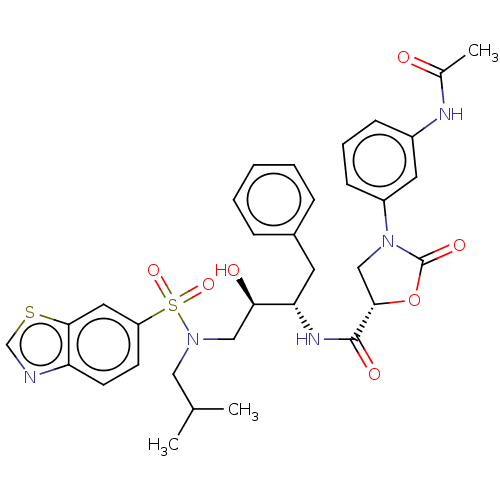 (CHEMBL1276096) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical School Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease by FRET | J Med Chem 53: 7699-708 (2010) Article DOI: 10.1021/jm1008743 BindingDB Entry DOI: 10.7270/Q2BZ68WM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM12885 ((5S)-N-[(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl[(4-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMBI Curated by ChEMBL | Assay Description Inhibition of HIV1 protease Q7K mutant by FRET method | J Med Chem 52: 737-54 (2009) Article DOI: 10.1021/jm8009525 BindingDB Entry DOI: 10.7270/Q2PN98FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50484193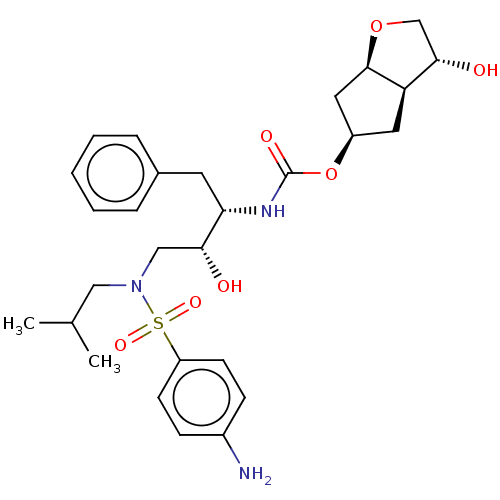 (CHEMBL1819294) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus 1 protease assessed as release of fluorescent N-terminal tripeptide using fluorogenic substrate by continu... | J Med Chem 54: 5890-901 (2011) Article DOI: 10.1021/jm200649p BindingDB Entry DOI: 10.7270/Q2DB84QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6879 total ) | Next | Last >> |