 Found 4 hits for monomerid = 20882
Found 4 hits for monomerid = 20882 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM20882
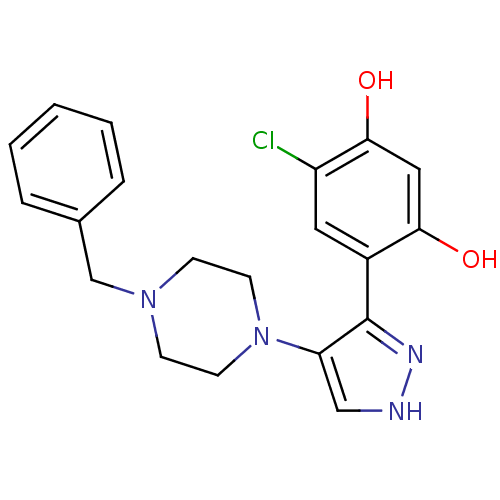
(4-[4-(4-benzylpiperazin-1-yl)-1H-pyrazol-3-yl]-6-c...)Show SMILES Oc1cc(O)c(cc1Cl)-c1n[nH]cc1N1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C20H21ClN4O2/c21-16-10-15(18(26)11-19(16)27)20-17(12-22-23-20)25-8-6-24(7-9-25)13-14-4-2-1-3-5-14/h1-5,10-12,26-27H,6-9,13H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | 6.50E+3 | n/a | n/a | 7.4 | 22 |
Vernalis (R&D) Ltd
| Assay Description
The assay is based upon displacement of a fluorescently labeled molecule, which binds specifically to the ATP-binding site of full-length human Hsp90... |
J Med Chem 51: 196-218 (2008)
Article DOI: 10.1021/jm701018h
BindingDB Entry DOI: 10.7270/Q2JW8C5C |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM20882

(4-[4-(4-benzylpiperazin-1-yl)-1H-pyrazol-3-yl]-6-c...)Show SMILES Oc1cc(O)c(cc1Cl)-c1n[nH]cc1N1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C20H21ClN4O2/c21-16-10-15(18(26)11-19(16)27)20-17(12-22-23-20)25-8-6-24(7-9-25)13-14-4-2-1-3-5-14/h1-5,10-12,26-27H,6-9,13H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Memorial Sloan-Kettering Cancer Center
Curated by ChEMBL
| Assay Description
Binding affinity to Hsp90 by fluorescence polarization assay |
Bioorg Med Chem 17: 2225-35 (2009)
Article DOI: 10.1016/j.bmc.2008.10.087
BindingDB Entry DOI: 10.7270/Q2T72JCS |
More data for this
Ligand-Target Pair | |
Heat Shock Protein 90 (Hsp90)
(Homo sapiens (Human)) | BDBM20882

(4-[4-(4-benzylpiperazin-1-yl)-1H-pyrazol-3-yl]-6-c...)Show SMILES Oc1cc(O)c(cc1Cl)-c1n[nH]cc1N1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C20H21ClN4O2/c21-16-10-15(18(26)11-19(16)27)20-17(12-22-23-20)25-8-6-24(7-9-25)13-14-4-2-1-3-5-14/h1-5,10-12,26-27H,6-9,13H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 by FP assay |
Bioorg Med Chem Lett 16: 2543-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.099
BindingDB Entry DOI: 10.7270/Q2G44PWS |
More data for this
Ligand-Target Pair | |
Heat Shock Protein 90 (Hsp90)
(Homo sapiens (Human)) | BDBM20882

(4-[4-(4-benzylpiperazin-1-yl)-1H-pyrazol-3-yl]-6-c...)Show SMILES Oc1cc(O)c(cc1Cl)-c1n[nH]cc1N1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C20H21ClN4O2/c21-16-10-15(18(26)11-19(16)27)20-17(12-22-23-20)25-8-6-24(7-9-25)13-14-4-2-1-3-5-14/h1-5,10-12,26-27H,6-9,13H2,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HSP90 ATPase activity |
Bioorg Med Chem Lett 16: 2543-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.099
BindingDB Entry DOI: 10.7270/Q2G44PWS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data