

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM22953 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1H-indol-3-yl}-N-(2-hydroxyethyl)acetamide::CHEMBL24415::Indomethacin derivative, 6
SMILES: COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)NCCO)c2c1
InChI Key: InChIKey=JHOVCAXRUUWNJL-UHFFFAOYSA-N
Data: 6 IC50
PDB links: 2 PDB IDs contain this monomer as substructures. 2 PDB IDs contain inhibitors having a similarity of 90% to this monomer.
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM22953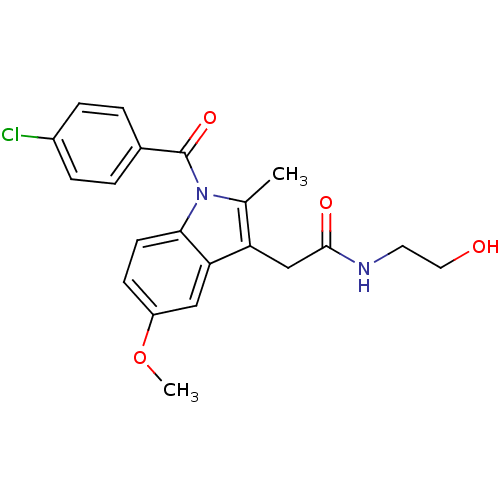 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >6.60E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Vanderbilt University School of Medicine | Assay Description For the time-dependent inhibition studies, COX enzyme was incubated with test compounds for 20 min and then analyzed for remaining COX activity by tr... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM22953 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | 37 |
Vanderbilt University School of Medicine | Assay Description For the time-dependent inhibition studies, COX enzyme was incubated with test compounds for 20 min and then analyzed for remaining COX activity by tr... | Proc Natl Acad Sci U S A 97: 925-30 (2000) Article DOI: 10.1073/pnas.97.2.925 BindingDB Entry DOI: 10.7270/Q2XP736G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM22953 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibitory concentration against human prostaglandin G/H synthase 2 | Bioorg Med Chem Lett 12: 1315-8 (2002) BindingDB Entry DOI: 10.7270/Q2S75FNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM22953 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against ovine Prostaglandin G/H synthase 1 (44 nM) using [14C]AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclooxygenase (Homo sapiens (Human)) | BDBM22953 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description In vitro inhibitory activity against human Prostaglandin G/H synthase 2 (66 nM) using [14C]-AA (50 uM) was determined | J Med Chem 43: 2860-70 (2000) BindingDB Entry DOI: 10.7270/Q22806VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM22953 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibitory concentration against prostaglandin G/H synthase 1 | Bioorg Med Chem Lett 12: 1315-8 (2002) BindingDB Entry DOI: 10.7270/Q2S75FNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||