

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: C[C@@H](NC(=O)c1cn2C[C@H]3[C@@H]4CC[C@@H](C4)N3C(=O)c2c(O)c1=O)c1c(F)cc(F)cc1F
InChI Key: InChIKey=KECPSAUYMNSAFH-ZEQKQHQNSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solute carrier family 22 member 2 (Homo sapiens (Human)) | BDBM330055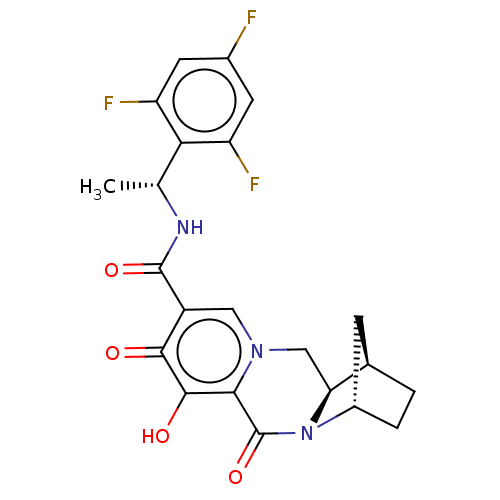 ((1R,4S,12aR)-7-hydroxy-6,8-dioxo-N—((R)-1-(2,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US9663528 (2017) BindingDB Entry DOI: 10.7270/Q24F1SVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| POU domain, class 2, transcription factor 2 (Homo sapiens (Human)) | BDBM330055 ((1R,4S,12aR)-7-hydroxy-6,8-dioxo-N—((R)-1-(2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description The dose dependent inhibition of OCT2 mediated uptake of a model substrate 14C-Tetraethylammonium (TEA) by test compounds was studied in wild-type an... | US Patent US10689399 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 2 (Homo sapiens (Human)) | BDBM330055 ((1R,4S,12aR)-7-hydroxy-6,8-dioxo-N—((R)-1-(2,...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Test compounds were serially diluted in DMSO and then spiked (2 μL) into in 0.4 mL KHB buffer containing wild-type or OCT2-transfected cells and... | US Patent US9732092 (2017) BindingDB Entry DOI: 10.7270/Q2SN0C2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||