

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN1CCN(CC1)C(=O)C1CC[C@@H](CC1)C(=O)Nc1cc2cc(ccc2cn1)-c1cnn(C)c1
InChI Key: InChIKey=LYTBFSXOMIMCTF-NZANSXMDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene Wnt-1 (Homo sapiens (human)) | BDBM422359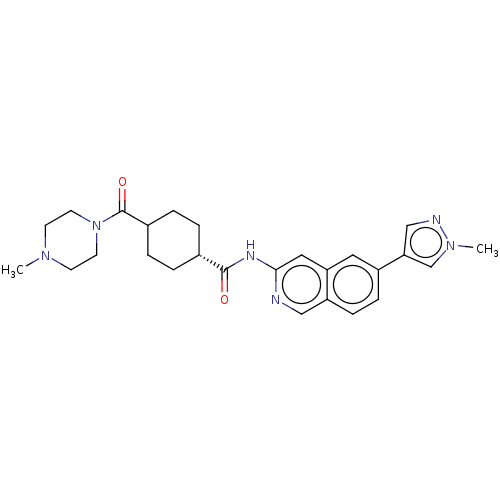 (US10508099, Compound 1091 | US10556885, Compound 1...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a |
Samumed, LLC US Patent | Assay Description SW480 colon carcinoma cells were transduced with a lentiviral vector expressing luciferase with a human Sp5 promoter consisting of a sequence of eigh... | US Patent US10508099 (2019) BindingDB Entry DOI: 10.7270/Q2DJ5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM422359 (US10508099, Compound 1091 | US10556885, Compound 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Samumed, LLC US Patent | Assay Description Each compound was dissolved in DMSO as a 10 mM stock and used to prepare compound source plates. Serial dilution (1:3, 11-point dose-response curves ... | US Patent US10508099 (2019) BindingDB Entry DOI: 10.7270/Q2DJ5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM422359 (US10508099, Compound 1091 | US10556885, Compound 1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 69 | n/a | n/a | n/a | n/a |
Samumed, LLC US Patent | Assay Description GSK3B: This is a non-radioactive assay using fluorescence resonance energy transfer (FRET) between coumarin and fluorescein to detect kinase activity... | US Patent US10556885 (2020) BindingDB Entry DOI: 10.7270/Q2ZW1P9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity tyrosine-phosphorylation-regulated kinase 1A (Homo sapiens (Human)) | BDBM422359 (US10508099, Compound 1091 | US10556885, Compound 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Samumed, LLC US Patent | Assay Description The DYRK1A kinase assay was run using the Ser/Thr 18 peptide Z-lyte assay kit according to manufacturer's instructions. This is a non-radioactive... | US Patent US10556885 (2020) BindingDB Entry DOI: 10.7270/Q2ZW1P9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM422359 (US10508099, Compound 1091 | US10556885, Compound 1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 69 | n/a | n/a | n/a | n/a |
Samumed, LLC US Patent | Assay Description Each compound is dissolved in DMSO as a 10 mM stock and used to prepare compound source plates. Serial dilution (1:3, 11-point dose-response curves f... | US Patent US10508099 (2019) BindingDB Entry DOI: 10.7270/Q2DJ5J0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||